Pharmacogenetic findings in anti-tuberculosis therapy: A study of the Latvian population
The aim of Viktorija Ulanova's doctoral thesis was to study the pharmacogenetic aspects of anti-tuberculosis therapy in the Latvian population, taking into account pharmacokinetic factors both individually and in combination, with a particular focus on isoniazid and aminoglycosides.
Personalised medicine tailors treatment to a patient's individual characteristics, such as genetics, environment, and lifestyle, and its main components are pharmacogenetics (PGx) and therapeutic drug monitoring (TDM). PGx studies how genetic variations affect the way drugs work and can optimise treatment and reduce the risk of adverse side effects. TDM involves measuring drug concentrations in biological samples to ensure effective and safe dosing. TDM is typically used for drugs with a narrow therapeutic index and significant individual differences in response to treatment. PGx and TDM provide clinically relevant information about the links between genetic variations and drug effects.
‘In this work, I am evaluating three key pharmacogenetic biomarkers of isoniazid metabolising enzymes and performing therapeutic drug monitoring tests to address inter-individual differences in isoniazid efficacy and toxicity. This is particularly important for patients with drug-sensitive TB. The study takes into account six key pharmacokinetic parameters of isoniazid and its two main metabolites, as well as MT-RNR11 gene variants associated with aminoglycoside-induced hearing loss (AIHL) in the treatment of multidrug-resistant tuberculosis. This broadens our understanding of the use of precision medicine in anti-tuberculosis therapy,’ says Ulanova, the author of the thesis.
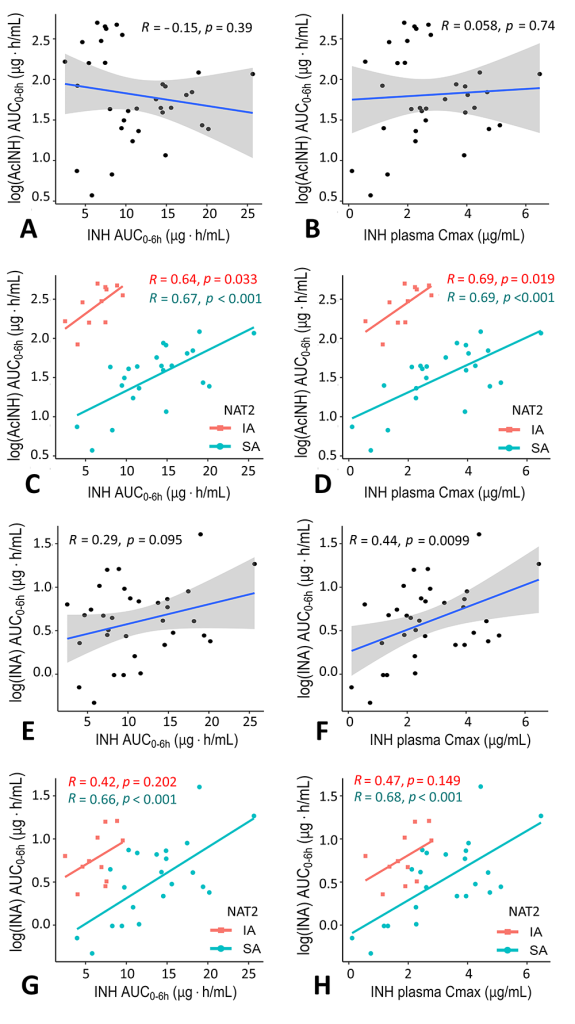
Correlation analysis results for isoniazid and two of its metabolites in blood plasma of tuberculosis patients.
The solid lines represent Pearson's correlation coefficient. The points represent the observed data for each patient.
INH – isoniazid
AcINH – acetylisoniazid
INA – isonicotinic acid
AUC – area under the concentration-time curve
Cmax – maximum concentration
NAT2 – N-acetyltransferase 2
SA – slow acetylator status
IA – intermediate acetylator status
The thesis had several objectives. First, to determine the frequency of NAT22 and GSTM13 genotypes in patients with drug-sensitive tuberculosis. Second, to develop a next-generation sequencing and bioinformatics-based protocol for full-length analysis of the CYP2E14 gene. The genetic determinants of key isoniazid metabolising enzymes and their association with pharmacokinetic parameters of isoniazid and its two metabolites were investigated to evaluate potential predictors of treatment response, treatment outcome, and the development of drug-induced hepatotoxicity in patients with drug-sensitive tuberculosis. Finally, the frequency of MT-RNR1 gene variants associated with AIHL was investigated in a ethnic Baltic language-speaking Latvian population and the distribution of these variants in mitochondrial haplogroups characteristic of the population was assessed.
Key findings
Next-generation sequencing technology can accurately identify CYP2E1 gene variants in a cost-effective manner and allows the simultaneous analysis of multiple gene regions.
All but one of the parameters of isoniazid and its metabolites were dependent on NAT2 acetylator status, while GSTM1 gene variants did not affect the results. The CYP2E1*6 variant was associated with isoniazid concentrations and metabolite ratios, indicating the utility of a combined genotype approach in PGx and TDM studies.
Genetic factors such as NAT2 acetylator status, GSTM1 genotype, and CYP2E1 gene variants were not associated with treatment outcome. However, several isoniazid parameters were associated with treatment efficacy, e.g. lower isonicotinic acid/isoniazid and acetylisoniazid/isoniazid ratios were associated with positive sputum cultures in the second month of treatment.
Genetic factors were not associated with isoniazid-induced liver injury, except for reduced plasma acetylisoniazid levels, which could serve as a biomarker for the diagnosis of isoniazid-induced liver injury.
The presence of six MT-RNR1 gene variants in the ethnic Latvian population indicates the need to include analysis of ototoxicity-related variants in future studies to evaluate the usefulness of mitochondrial DNA screening in patients before starting aminoglycoside therapy.
Next steps
The inclusion of isoniazid and its metabolites in therapeutic drug monitoring using three time points could help to accurately predict drug concentrations and develop optimal dosing strategies.
The study results showed that parameters of isoniazid and its metabolites were dependent on NAT2 acetylator status, and reduced acetylisoniazid levels were associated with isoniazid-induced liver damage, highlighting the importance of genetic variation in relation to drug efficacy.
The presence of MT-RNR1 gene variants in the ethnic Latvian population highlights the need for future studies to include analysis of ototoxicity-associated variants to assess the suitability of DNA screening of patients prior to initiation of aminoglycoside therapy.
Further research is needed to assess whether allelic variants of enzymes involved in drug metabolism confer a risk of inadequate response to drugs used to treat tuberculosis and increased susceptibility to adverse drug reactions in patients with different genetic backgrounds.
1 MT-RNR1 – gene encoding the mitochondrial ribosomal subunit 12S
2 NAT2 – N-acetyltransferase 2
3 GSTM1 – glutathione S-transferase M1 class
4 CYP2E1 – cytochrome P450 family 2 subfamily E member 1
Related news
 RSU research project on early diagnosis of bladder cancer receives recognition from the Latvian Academy of SciencesRecognition, Research, Innovation
RSU research project on early diagnosis of bladder cancer receives recognition from the Latvian Academy of SciencesRecognition, Research, Innovation