Food Allergy and Food Hypersensitivity in Children 0–3 years
Abstract
Food allergy (FA) is a rising problem worldwide. No previous studies of FA and food hypersensitivity have been conducted in Latvia.
The objectives of the respective research include studies of the symptoms that are supposed to be caused by FA and identification of food allergens and tests used in diagnostics of FA in different age groups in children up to three years of age.
Data for the retrospective descriptive study were obtained from randomly sampled medical documents from allergy outpatient care in Children’s Clinical University Hospital in Rīga. Children, 0–3 years old, participated in this study, divided into the following age groups: 0–6 months, 6–12 months, 12–18 months, 18–24 months and 2–3 years. For statistical analysis, MS Excel and IBM Statistics 20.0 programs were used.
Data from 100 children medical documents were collected; 61 % boys, 39 % girls. 65 % of all patients were infants. In 92 % of patients, symptoms had started in the first year of life. Patients most commonly had positive tests to egg and milk (71 % and 40 %, respectively). Specific IgE tests were used in 61 % patients, skin prick tests in 51 %, atopy patch tests (in combination with prick tests or specific IgE) in 20 %. Oral food challenges were not performed. The most common complaints were skin symptoms (98 %) and gastrointestinal problems (10 %). 94 % (n = 94) had atopic dermatitis, 3 % (n = 3) anaphylactic reactions, 2 % (n = 2) respiratory symptoms. 12 % of patients had symptoms from more than one organ system.
This study has revealed that egg and milk are the most common food allergens found in children in the first three years of life. Skin problems could be the most worrying complaint for parents, which might explain why almost all patients who participated in this study had skin problems. Gastrointestinal symptoms are poorly recognised and evaluated as a sign of food allergy. Oral food challenges are necessary tests for diagnosis of food allergy and must be conducted in Children’s Clinical University Hospital in Rīga.
Introduction
Food allergy (FA) is a rising health concern that affects both children and adults. Food hypersensitivity may be the first stage in the development of allergic diseases such as atopic eczema [Bath-Hextall, 2009]. The highest FA incidence occurs during the first year of life [Fiocchi, 2011], around the age of 6–9 months [Bath-Hextall, 2009].
The Guidelines for the Diagnosis and Management of Food Allergy in the United States: Report of the NIAID-Sponsored Expert Panel define FA as an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food [Bergmann, 2013]. It also indicates that sensitisation (as evidenced by the presence of allergen-specific immunoglobulin E (sIgE)) to food allergens alone is not sufficient to define FA [Boyce, 2010].
Existing studies of the incidence, prevalence and natural history of FA are difficult to compare owing to inconsistencies and deficiencies in study design and variations in the definition of FA [Alle, 2012]. Many studies define food allergies based on history, which is inaccurate. Other studies use IgE positivity or skin test positivity to specific foods as a marker of FA. Between 50 % and 75 % of patients with sIgE to food tolerate the food [Lack, 2008]. The prevalence of food allergies has been estimated to be 5–6 % in infants and children younger than 3 years [Sicherer, 2013].
More than 170 foods have been reported to cause IgE-mediated reactions [Boyce, 2010]. Milk, egg, peanut, tree nuts, fish, shellfish, wheat and soy are considered to cause most of the food adverse reactions [Ito, 2012]. Cow’s milk protein together with hen’s egg protein are the key triggers of food allergy in infants and young children [Koletzko, 2012].
Clinical picture of food allergy is largely dependent on the pathogenesis (IgE or non-IgE response), patient’s age and organ systems involved [Lozovskis, 2009]. The immune reaction may be IgE-mediated, non-IgE mediated, or mixed [Koletzko, 2012]. IgE-mediated reactions are characterised by an acute onset of symptoms generally within 2 hours after the ingestion of or exposure to the trigger food; they typically involve the skin, gastrointestinal tract, and respiratory tract [Burks, 2012]. Mixed IgE and non-IgE mediated reactions are atopic dermatitis, eosinophilic esophagitis and eosinophilic gastroenteritis. Food protein-induced enterocolitis syndrome, food protein-induced allergic proctocolitis, allergic contact dermatitis, Heiner syndrome are caused by non-IgE mechanisms [Burks, 2012]. Food generally appears to be the most common trigger of anaphylaxis in the community [Sicherer, 2011]. Reactions can occur following ingestion, inhalation or contact with foods [Fiocchi, 2011].
Double blind placebo controlled food challenge (DBPCFC) is the gold standard of FA diagnosis. However, a single-blind or an open-food challenge may be considered diagnostic under certain circumstances [Boyce, 2010]. The European Academy of Allergology and Clinical Immunology considers that the involvement of subjective factors is negligible among infants and children younger than three years of age and has approved the use of open food challenges in the diagnosis of FA [Yang, 2012]. Other frequently used tests are skin prick tests (SPT), detection of sIgE, atopy patch tests (APT).
The potential severity of the disease and the specific public health measures required for FA make it important to identify the specific risk factors for this condition [Cochrane, 2009]. The primary therapy for FA is strict avoidance of the causal food or foods [Burks, 2012]. However, such diets might induce nutritional deficiencies if applied indiscriminately and without a clear indication [Bergmann, 2013]. A diet that is not indicated or continued when the child may have already developed tolerance may impair growth and quality of life of both child and family, while incurring significant unnecessary health care costs [Koletzko, 2012].
Most children with FA eventually will tolerate milk, egg, soy, and wheat; far fewer will eventually tolerate tree nuts and peanut. The time course of FA resolution in children varies by food and may occur as late as teenage years [Boyce, 2010]. 90 % of infants who are allergic to cow’s milk may tolerate it by the end of their third year, whilst half their peers who are allergic to egg do not react to it at the same age [Fiocchi, 2011].
Aim
The aim of the research is to study the symptoms that are supposed to be caused by FA, identify food allergens and tests used in diagnostics of FA in different age groups in children up to three years of age.
Material and methods
It was a retrospective descriptive study. The study was conducted in Children’s Clinical University Hospital in Rīga. Children 0–3 years old with suspected FA participated in this study. All patients were divided into the following age groups: 0–6 months, 6–12 months, 12–18 months, 18–24 months and 2–3 years. The group selection was based on the differences in children’s feeding habits and the fact that FA usually starts in infancy and in most cases tolerance to food is achieved up to the age of three years.
Data from randomly sampled medical documents from allergy outpatient care in Children’s Clinical University Hospital in Rīga in year 2012 were collected for patients with FA diagnosis or positive allergy tests (sIgE, SPT, APT to food allergens or positive oral food challenge (OFC) tests). Evaluation was done concerning patients sex, age at the time of consultation and time of the onset of symptoms, and the type of symptoms in different age groups. Food allergens to which patients had positive allergy tests were assessed in total and in each age group. Cow’s milk, egg, wheat, soy, nuts/peanuts, fish/crustaceans were counted separately, but other food allergens were put into one group since they were not among the most common food allergens. These included potato, carrot, banana, kiwi, broccoli, sweet pepper, tomato, pumpkin, apple, rye, oat, rice, chicken, pork and cacao. The evaluation was also done for the diagnostic methods used in study patients – SPT, sIgE, APT, OFC tests, diagnosis based on history and physical examination and other methods in each age group and in total.
Exclusion criteria: patients with celiac disease or other known gastrointestinal diseases, growth retardation due to a disease other than food allergy, airway diseases (other than bronchial asthma, allergic rhinitis, not associated with allergy), previously diagnosed skin diseases (except atopic dermatitis) or other known diseases with symptoms that resemble food allergy.
For statistical analysis, MS Excel and IBM Statistics 20.0 programs with comparative and descriptive methods were used with confidence interval (CI) 95 %.
Results
Data from 100 children medical documents were collected. 61 % (n = 61) were boys, 39 % (n = 39) were girls. 65 % (n = 65) of all patients were infants (0–6 months 31 %, n = 31; 6–12 months 34 %, n = 34). The number of patients in other age groups was as follows: 12–18 months 20 patients (20 %), 18–24 months 6 patients (6 %), 2–3 years 9 patients (9 %). In 92 % (n = 92) of cases symptoms had started in the first year of life. There was no information about the time of the onset of symptoms in five medical cards.
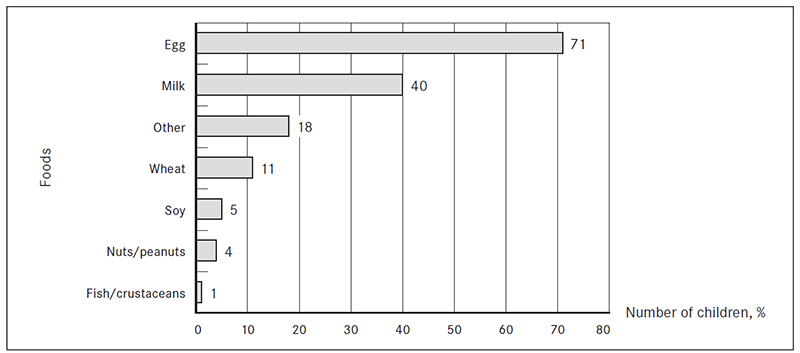
Food allergens. Egg was the most common food allergen among the study patients and the hypersensitivity to egg differed significantly from the second most common food allergen – milk (z score 4.53). Hypersensitivity to egg was found in 71 % (n = 71) and to milk in 40 % (n = 40) of patients. Hypersensitivity to other food allergens was as follows: wheat 11 % (n = 11), soy 5 % (n = 5), nuts/peanuts 4 % (n = 4), fish/crustaceans 1 % (n = 1) and other foods 18 % (n = 18) (Figure 1).
Figure 1. The most common food allergens in children 0–3 years of age (% from the total number of patients)
There was no statistically significant association between the age of the patient and possible food allergen. The overall occurrence of each food allergen in age groups is shown in Table 1.
In 40 % (n = 40) of cases hypersensitivity to several food allergens was detected; furthermore, it was more often seen in the first year of life – 25 % (n = 10) of infants 0–6 months and 43 % (n = 18) of infants 6–12 months of age. 15 % (n = 6) of children in age group 12–18 months and 8 % (n = 3) of children in both age group 18–24 months and age group 2–3 years were sensitised to more than one food allergen.
Diagnostic methods. In 5 % (n = 5) of patients food allergy was diagnosed based on medical history and physical examination. Two of these patients had had anaphylactic reactions in the past (to nuts and broccoli).
SPT and evaluation of sIgE were statistically significant the most commonly used diagnostic methods in the study patients (z score 9.23). SPT (alone or in combination with other tests) were used in 51 % (n = 51) of patients, sIgE (alone or in combination with other tests) in 61 % (n = 61) of patients. APT (in combination with sIgE or SPT) were used in 20 % (n = 20). OFC were not done (Figure 2).
The most common diagnostic methods used in each age group were as follows: in age group 0–6 month detection of sIgE (32 %, n = 10), in age group 6–12 months SPT (41 %, n = 14), in age group 12–18 months detection of sIgE (40 %, n = 8), in age group 18–24 months and 2–3 years SPT (50 %, n = 3 and 44 %, n = 4).
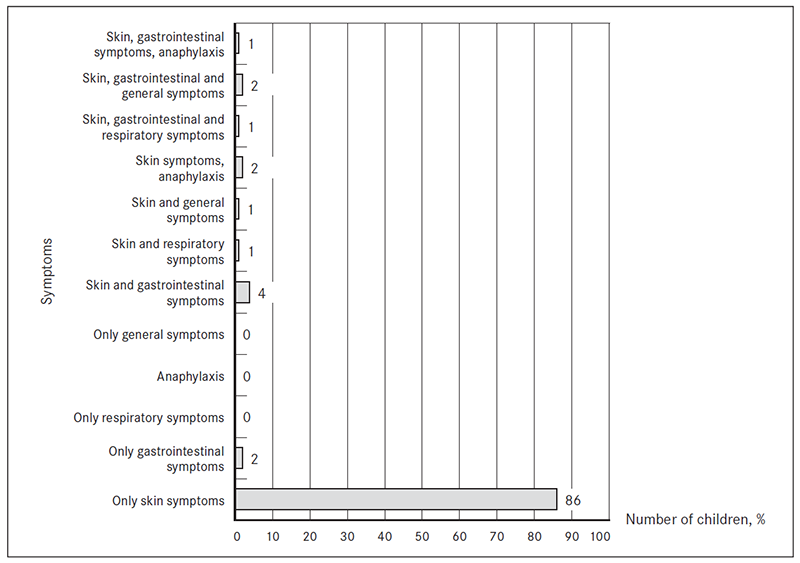
Symptoms. The most common complaints were skin symptoms (98 %, n = 98) and gastrointestinal (GI) problems (10 %, n = 10). Anaphylactic reactions were observed in 3 % (n = 3), respiratory symptoms in 2 % (n = 2), general symptoms (growth failure, irritability) in 3 % (n = 3). 12 % (n = 12) of patients had symptoms from more than one organ system. Only two patients had isolated GI symptoms. None of the patients had respiratory symptoms as the only complaint (Figure 3).
The skin symptoms were as follows: 96 % (n = 96) of patients had atopic dermatitis, 6 % (n = 6) had urticaria, 1 % (n = 1) had other skin eruptions. Three patients had several skin symptoms.
50 % (n = 5) of patients with GI symptoms had regurgitation or vomiting, three patients had loose stool/ diarrhoea, two patients had constipation. Blood and/or mucus in the stool was a complaint for one patient.
Skin symptoms were statistically the most significant common complaint in the study patients and their occurrence differed statistically significantly from GI disorders which were the second most common complaint (z score was 12.49 at the level of significance 0.05 (or 95 % probability).
Atopic dermatitis was the most common symptom in all age groups.
Table 1. The comparison of food allergens in different age groups (% of patients in age groups)
| Allergens | Age groups | ||||
|---|---|---|---|---|---|
| 0–6 months (n = 31) | 6–12 months (n = 34) | 12–18 months (n = 20) | 18–24 months (n = 6) | 2–3 years (n = 9) | |
| Percentage (%) and number (n) of patients in the age group | |||||
| Egg | 74 % (n = 23) | 68 % (n = 23) | 70 % (n = 14) | 67 % (n = 4) | 78 % (n = 7) |
| Milk | 39 % (n = 12) | 56 % (n = 19) | 35 % (n = 7) | 17 % (n = 1) | 11 % (n = 1) |
| Wheat | 10 % (n = 3) | 12 % (n = 4) | 10 % (n = 2) | 17 % (n = 1) | 11 % (n = 1) |
| Soy | 3 % (n = 1) | 9 % (n = 3) | 5 % (n = 1) | 0 | 0 |
| Nuts/peanuts | 3 % (n = 1) | 0 | 0 | 33 % (n = 2) | 11 % (n = 1) |
| Fish/crustaceans | 0 | 3 % (n = 1) | 0 | 0 | 0 |
| Other | 10 % (n = 3) 21 | 21 % (n = 7) | 20 % (n = 4) | 17 % (n = 1) | 33 % (n = 3) |
Figure 2. Diagnostic methods of FA and food hypersensitivity used in studied patients (% of total, n = 100)
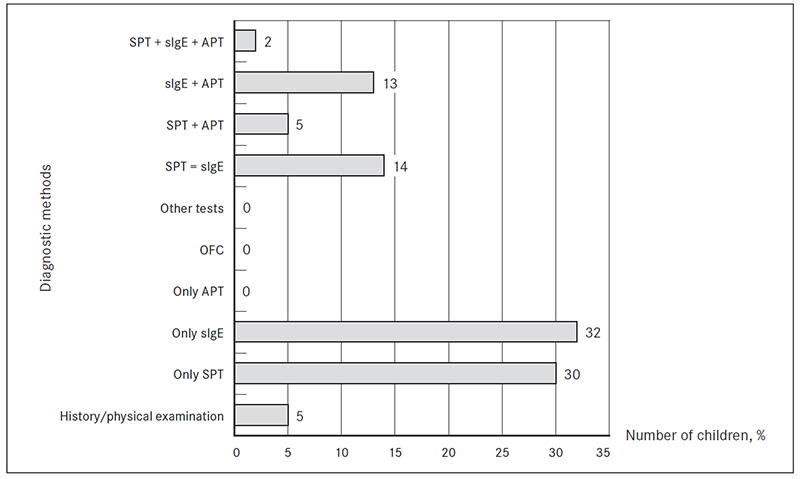
Figure 3. Symptoms in which FA were suspected (% of patients from total, n = 100)
Discussion
FA is a rising health concern worldwide that mostly affects young children but also can persist in adulthood. The highest FA incidence occurs during the first year of life [Fiocchi, 2011]. The results of this study revealed that most of the patients with suspected FA and diagnosed hypersensitivity to food allergens were infants. It is often discussed that milk is the most common food allergen in children. Several studies indicate that allergy and hypersensitivity to egg in infancy is more common than to other food allergens [Fiocchi, 2010; Salehi, 2009]. The results of The Early Prevention of Asthma in Atopic Children (EPAAC TM) study show that egg is the most important food allergen in children with atopic dermatitis in the first two years of life [Benedictis, 2009]. Egg was the most common food allergen among patients of this study and the hypersensitivity to egg differed significantly from the second most common food allergen – milk. Hypersensitivity to other food allergens in patients of this study was observed less frequently.
The US FA guidelines of 2010 [Boyce, 2010] advise that the nature of the reaction often suggests the underlying mechanism, either IgE-mediated (immediate) or non-IgE mediated (delayed), and will determine the diagnostic tests to be used. For detection of IgE-mediated reaction SPT and evaluation of sIgE antibodies are used. It is important to notice that neither SPT nor detection of sIgE when used alone are diagnostic of FA [Boyce, 2010]. In diagnosing FA or food hypersensitivity in patients of our study SPT or sIgE test were performed most often. SPT are easy to perform and the results are ready after 15 minutes. On the other hand, this test cannot be done on damaged skin or when antihistamines or systemic steroids are being used. Mehl, et al. study shows that the concordance between SPT and sIgE is surprisingly low for cow’s milk and hen’s egg on individual basis. In children who receive a negative test result the alternative test should also be used [Mehl, 2012]. A number of studies report that the APT may be useful in the evaluation of food allergy in patients with atopic dermatitis and eosinophilic eosophagitis [Boyce, 2010]. The US FA guidelines of 2010 indicate that insufficient evidence exists to support the use of the APT for the evaluation of food allergy. None of the patients in this study had APT performed as the only test for evaluation of possible FA. Negative SPT and/or sIgE test would explain why APT was done, since this test was generally used to assess delayed or non-IgE mediated reactions to a food allergen. Unfortunately, DBPCFC, which is the gold standard test for FA diagnosis was not performed for the study patients. This item should be seriously taken into consideration because without these tests FA diagnosis can be established only in several cases, for example, anaphylactic reactions after specific food and positive SPT for this food.
By evaluating the symptoms of the study patients, three of the patients reported anaphylactic reactions. Two of these patients had reaction to two of the most possible foods that cause anaphylactic reactions – nuts and fish. It was well seen that skin symptoms, particularly atopic dermatitis, was the most common problem of the study patients. Only two patients had isolated GI symptoms. Approximately 40 % of infants and young children with moderate to severe atopic eczema have FA [Fiocchi, 2011]. It is indicated that 50–60 % of children with cow milk allergy have GI symptoms [Vandenplas, 2007; Greef, 2012], and these symptoms are found in 41 % of children with soy allergy [Savage, 2010]. Since there were no OFC performed for these study patients, it is difficult to link food allergens with symptoms, we can only discuss possible food hypersensitivity, not real FA. On the other hand, it was noticeable that FA was more often suspected in patients with skin problems. GI symptoms might be underestimated as potential symptoms for FA.
Conclusions
- 92 % of patients with suspected food allergy have developed symptoms till 12 months of age. 65 % of patients at the time of consultation were infants.
- Egg is statistically the most significant common food allergen among the study patients and hypersensitivity to egg differs statistically significantly from the hypersensitivity to the second most common food allergen – milk.
- There is no statistically significant association between the age of the patient and possible food allergen.
- Most frequently used diagnostic methods are skin prick tests or detection of sIgE antibodies.
- Oral food challenges are not done, because they are time consuming and require specific preparation.
- Skin problems are statistically the most significant common complaints for the study patients, the second most common complaint – gastrointestinal symptoms.
- Gastrointestinal symptoms are not well recognised as a possible clinical manifestation of food allergy.
- For diagnosis of food allergy and prescription of adequate elimination diet in Children’s Clinical University Hospital in Rīga, it is necessary to perform oral food challenge tests.
Acknowledgement
The study was conducted as a resident practical scientific research at Rīga Stradiņš University Faculty of Continuing Education and as a part of Dr. Elina Aleksejeva’s dissertation “The role of atopy patch tests in diagnostics of food allergy in children with atopic dermatitis and gastrointestinal symptoms”.
References
- Alle K. J., Koplin J. J. The Epidemiology of food allergy // Food allergy / Ed. by James J. M. – 1st ed. – China: Elsevier Inc., 2012. – Pp. 309–312.
- Barry P., Brown T., Beattie P., et al. National Institute for Health and Clinical Excellence (2011). Diagnosis and assessment of food allergy in children and young people in primary care and community settings. – London: National Institute for Health and Clinical Excellence. 2011 // www.nice.org.uk/guidance/CG116 (viewed 2013.02.10).
- Bath-Hextall F. J., Delamere F. M. Williams H. C. Dietary exclusions for improving established atopic eczema in adults and children: Systemic review // J Allergy 2009; 64: 258–264.
- Battais F., Richard C., Jacquenet S., Denery-Papini S. Wheat grain allergies: An update on wheat allergens // Eur Ann Allergy Clin Immunol, 2008; 409 (3): 67–76.
- Benedictis F. M., Franceschini F., Hill D., et al. The allergic sensitization in infants with atopic eczema from different countries // J Allergy, 2009; 64: 295–303.
- Bergmann M. M, Caubet J. C., Boguniewicz M., Eigenmann P. A. Evaluation of food allergy in patients with atopic dermatitis // J Allergy and Clin Immunol: In Practice, 2013; 1: 22–28.
- Bo Y. C., Hye O. K., Chun W. P., Cheol H. L. Diagnostic usefulness of the serum-specific IgE, the skin prick test and the atopy patch test compared with that of the oral food challenge test // Ann Dermatol, 2010 November; 22 (4): 404–411.
- Boyce J. A., Assa’ad A., Burks A. W., et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel // J Allergy and Clin Immunol, 2010; 126: 1–58.
- Burks A. W., Jones S. M., Boyce J. A., et al. NIAID-sponsored 2010 Guidelines for managing food allergy: Applications in the pediatric population // J Pediatrics, 2011; 128: 955–965.
- Burks A. W., Tang M., Sicherer S., et al. ICON: Food allergy // J Allergy and Clin Immunol, 2012; 129: 906–920.
- Caubet J. C., Wang J. Current understanding of egg allergy // Pediatr Clin North Am, 2011 April 1; 58 (2): 427–443.
- Cochrane S., Beyer K., Clausen M., et al. Factors influencing the incidence and prevalence of food allergy // J Allergy, 2009; 64: 1246–1255.
- Cudowska B., Kaczmarski M. Atopy patch test in the diagnosis of food allergy in children with gastrointestinal symptoms // J Adv Med Sci, 2010; 55: 153–160.
- Fiocchi A., Brozek J., Schünemann H., et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines // WAO Journal, 2010 April.
- Fiocchi A., Sampson H. A., Bahna S. L., et al. Food allergy // WAO White Book on Allergy / Ed. by Pawankar R. – Copyright UK: World Allergy Organization, 2011. – Pp. 47–52.
- Kelly C., Gangur V. Sex disparity in food allergy: Evidence from the PubMed database // J Allergy, 2009 (2009): 1–7.
- Greef E., Hauser B., Devreker T., et al. Diagnosis and management of cow’s milk protein allergy in infants // World J Pediatr, 2012; 8 (1): 19–24.
- Guandalin S., Nocerino A., Vargas J. H., et al. Soy protein intolerance // www.emedicine.medscape.com/article/932026-overview#a0199 (viewed 2013.10.03).
- Hong X., Tsai H. J., Wang X. Genetics of food allergy // Curr Opin Pediatr, 2009 Dec; 21 (6): 770–776.
- Ito K., Futamura M., Movérare R., et al. The usefulness of casein-specific IgE and IgG4 antibodies in cow’s milk allergic children // J Clinical Molecular Allergy, 2012, 10 (1): 1–6.
- Kneepkens C. M. F., Meijer Y. Clinical practice. Diagnosis and treatment of cow’s milk allergy // Eur J Pediatr, 2009; 168: 891–896.
- Koletzko S., Niggemann B., Arato A., et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee Practical Guidelines // JPGN, 2012; 55: 221–229.
- Lack G. Epidemiologic risks for food allergy // J Allergy and Clin Immunol, 2008; 121:1331–1336.
- Lee L. A. Atopic dermatitis and allergy in children: A dynamic relationship // J Food Chemical Toxicology, 2008; 46: 6–11.
- Palena S, Sebre D., Grantiņa I. Uztura alerģija [eng. Food allergy] // Lozovskis V. Praktiskā alergoloģija. – Rīga: LU Akadēmiskais apgāds, 2009. – Pp. 473–501.
- Mehl A., Niggemann B., Keil T., et al. Skin prick test and specific serum IgE in the diagnostic evaluation of suspected cow’s milk and hen’s egg allergy in children: Does one replace the other? // J Clinical Experimental Allergy, 2012; 42: 1266–1272.
- Osborne N. J., Koplin J. J., Martin P. E., et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants // J Allergy and Clin Immunol, 2011; 127: 668–676.
- Pierett M. M., Järvinen K. M. The natural history of wheat allergy // J Pediatrics, 2009; 124: S121.
- Pustisek N., Jaklin-Kekez A., Frkanec R., et al. Our experiences with the use of atopy patch test in the diagnosis of cow’s milk hypersensitivity // J Acta Dermatovenerol Croat, 2010; 18 (1): 14–20.
- Salehi T., Pourpak Z., Karkon S., et al. The study of egg allergy in children with atopic dermatitis // WAO Journal, 2009; 2: 123–127.
- Savage J. H., Kaeding A. J., Matsui E. C., Wood R .A. The natural history of soy allergy // J Allergy and Clin Immunol, 2010; 125: 683–686.
- Sicherer S. H. Epidemiology of food allergy // J Allergy and Clin Immunol, 2011; 127: 594–602.
- Sicherer S. H., Kaliner M. A., Atkins D., et al. Food allergies // www.emedicine.medscape.com/article/135959-overview (viewed 2103.01.25).
- Stapel S. O., Asero R., Ballmer-Weber B. K., et al. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report // J Allergy, 2008; 63: 796.
- Suh J., Lee H,. Lee J. H., et.al Natural course of cow’s milk allergy in children with atopic dermatitis // J Korean Med Sci, 2011; 26: 1152–1158.
- Syrigou E. I., Pitsios C., Panagiotou I., et al. Food allergy-related paediatric constipation: The usefulness of atopy patch test // Eur J Pediatr, 2011; 170; 1173–1178.
- Tan T. H. T., Ellism J. A., Saffery R., Allen K. J. The role of genetics and environment in the rise of childhood food allergy // Clinical Experimental Allergy, 2012; 42: 20–29.
- Vandenplas Y., Brueton M., Dupont C., et al. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants // Arch Dis Child, 2007; 92: 902–908.
- Werfel T., Erdmann S., Fuchs T., et al. Approach to suspected food allergy in atopic dermatitis. Guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA) // J German Soc Dermatol, 2009; 3 (7): 265–271.
- Yang H., Xiao Y. Z., Luo X. Y., et al. Diagnostic accuracy of atopy patch tests for food allergy in children with atopic dermatitis aged less than two years // www.elsevier.es/eop/S0301-0546(12)00272-8.pdf (viewed 2013.02.23).
- Wahn U. The allergic march // www.worldallergy.org/professional/allergic_diseases_center/allergic_march/ (viewed 2013.03.20).