Human Herpesvirus 6 and Parvovirus B19 Infection in Patients with Chronic Autoimmune Thyroiditis and in Practically Healthy Blood Donors
Abstract
The human pathogenic beta-herpesvirus 6 (HHV-6) and parvovirus B19 (B19) are immunomodulating viruses that are unique due to broad cell tropism including thyroid tissue. HHV-6 and B19 are triggers of many autoimmune diseases and have been associated with autoimmune thyroid gland diseases. Autoimmune thyroid gland diseases are multifactorial diseases and different factors have been proposed to contribute to their aetiology; however, the primary cause is still unknown.
This work aimed to estimate the frequency of HHV-6 and B19 infection in patients with chronic autoimmune thyroiditis and practically healthy blood donors. 60 patients with struma nodosa after thyroidectomy and 60 practically healthy blood donors were included in this study. Thyroid-specific autoantibodies against thyroid peroxidase (TPOAb), TSH receptor (TRAb) and thyroglobulin (TGAb) were detected using ELISA. HHV-6 and B19 genomic sequence in DNA extracted from whole blood and cell-free plasma was detected using nested polymerase chain reaction (nPCR).
High titre of thyroid-specific autoantibodies was detected significantly more often in patients with thyroid gland pathologies than in practically healthy donors (27/60 vs. 0/59; p = 0.0001). HHV-6 genome sequence was found in 15 out of 27 (55.5 %) patients with chronic autoimmune thyroiditis which is significantly more often comparing to the practically healthy donors – 8 out of 59 (13.5 %) (p = 0.0001). HHV-6 genome sequence was found also in 1 out of 5 (20.0 %) patient with chronic thyroiditis without autoimmune component and in 18 out of 28 (64.2 %) patients without chronic thyroiditis. B19 genome sequence was found significantly more often in patients with chronic autoimmune thyroiditis – 25.9 % (7 out of 27) in comparison with practically healthy donors 6.7 % (4 out of 59; p = 0.031). However, B19 genome sequence was detected also in one patient with chronic thyroiditis without autoimmune component and 10 out of 28 patients without chronic thyroiditis. No statistical significance was observed in virus frequency between patients according to high or normal range of thyroid-specific autoantibodies.
The high detection rate of HHV-6 and B19 infection suggest a potential pathogenic role of these viruses in development of thyroid gland diseases.
Introduction
Autoimmune thyroiditis is a common disease characterised by lymphocytic infiltration and the presence of thyroid-specific autoantibodies. Despite the intensive investigation of chronic inflammatory and autoimmune processes, there are many uncertainties so far concerning their aetiological factors. Viral infections are frequently cited as a major environmental factor involved in thyroid gland diseases [Prumel, 2004]. Several reports have provided an important information linking HHV-6 to autoimmune diseases including multiple sclerosis, autoimmune connective tissue diseases and Hashimoto’s thyroiditis [Nora-Krukle, 2011; Broccolo, 2013; Caselli, 2012]. In 1986, Ablashi, Gallo and Salahuddin discovered human herpesvirus 6 (HHV-6), a member of the Roseolovirus genus, Herpesviridae family, β-herpesvirus subfamily [Ablashi, 1987]. HHV-6 is lymphotropic, immunomodulating virus characterised by widespread tissue tropism and has been found also in the thyroid gland tissue. Infection with HHV-6 is a global concern, and it is the aetiological agent of many diseases. However, little literature on HHV-6 involvement in thyroiditis is available. An excellent review has been published where researchers from Italy have shown that HHV-6 infection may play a role in the pathogenesis of Hashimoto’s thyroiditis [Caselli, 2012]. Human parvovirus B19 (B19) is the member of the family Parvoviridae and is a cause of a childhood exanthema, but it has also been well documented that B19 is a common human pathogen, which has been linked to autoimmune diseases such as autoimmune neutropenia, thrombocytopenia, haemolytic anaemia and rheumatoid arthritis [Desailloud, 2009]. During the last few years, studies have suggested the association of B19 infection with autoimmune thyroiditis [Wang, 2010; Page, 2014]. According to Mori and his colleagues review, intra-thyroidal persistence of B19 DNA in a patient with Hashimoto’s thyroiditis has been detected. The cell types responsible for the B19 DNA persistence are not determined and immune cells infiltrating the thyroid gland may be the source of B19 DNA. However, the possibility that thyroid epithelial cells harbour B19 DNA cannot be excluded [Mori, 2007].
Aim
This study aimed to estimate the frequency of HHV-6 and B19 infection in patients with chronic autoimmune thyroiditis and practically healthy blood donors.
Materials and methods
All laboratory tests were approved by the Ethical Committee of Rīga Stradiņš University (Rīga, Latvia). Patients with struma nodosa thyreotoxicum or euthyreosis were referred to thyroidectomy and patients’ material was received after thyroidectomy performed at Rīga Eastern Clinical University Hospital, Clinic “Gaiļezers”. In total 60 patients: 32 patients with chronic thyroiditis (27 of them with chronic autoimmune thyroiditis) and 28 patients without markers of chronic thyroiditis were included in this study. The exact diagnosis of the thyroid gland pathology was established by histological analysis of resected tissue and analysis of thyroid gland specific autoantibodies. The age of the patients ranged from 40 to 78 (mean age – 50) and only 5 of them were men. 60 age-matched practically healthy blood donors were included as the control group. Blood plasma was separated from the EDTA whole blood, aliquoted and stored at −70 °C. DNA was isolated from 0.5 ml of whole blood and 0.2 ml cell-free plasma by proteinase K digestion followed by phenol-chloroform extraction and ethanol precipitation. The plasma samples were treated with DNase1 before DNA purification. B-globin PCR was performed in order to assess quality of the extracted DNA [Vandamme, 1995]. Negative b-globin PCR result for DNA isolated from plasma shows that there is no presence of cell DNA in the sample. Nested polymerase chain reaction (nPCR) with the corresponding primer pairs was used for the detection of B19 and HHV-6 genomic sequences [Feraj, 2001; Secchiero, 1995]. DNA samples negative for B19 and HHV-6 specific sequences and water controls were included in each experiment to exclude the possibility of contamination during PCR. The amplified DNA was analysed in 1.7 % agarose gel with ethidium bromide staining and analysed using BioSpectrum Imaging System. Autoantibodies against thyroid peroxidase (TPOAb), thyroglobulin (TGAb) and thyroid stimulating hormone (TRAb) were detected using ELISA kit (Euroimmun).
The data were analysed using Fisher’s test and Student’s t test.
Results
Statistical analysis showed no significant difference in age and gender between the patients and control group individuals. Plasma samples from 60 patients with struma nodosa after thyroidectomy and 60 practically healthy blood donors were investigated for thyroid-specific autoantibodies against TPOAb, TRAb and TGAb using the corresponding Euroimmun Immunoassays. 27 out of 60 (45 %) patients had high titre of TRAb (2.0 – 40.0 ± 13.07 IU/ml); TGAb (107.0 – 1070.0 ± 312.68 IU/ml) and TPOAb (90.0 – 500.0 ± 164.13 IU/ml). 33 (55 %) patients were within the normal range of TRAb (< 1.8 IU/ml) and TGAb (< 100 IU/ml) and TPOAb (< 50 IU/ml) (Figure 1).
One individual was excluded from the control group because of high titre of autoantibody against TPO (395.0 IU/ml) (suspected patient of chronic autoimmune thyroiditis).
The presence of HHV-6 and B19 was analysed in whole blood and cell-free plasma DNA samples from all patients with and without chronic thyroiditis and control group individuals according to the titres of thyroid specific autoantibodies. HHV-6 specific genomic sequence was detected in 34 out of 60 (56.6 %) whole blood DNA samples from patients: 15 out of 27 (55.5 %) whole blood DNA samples from patients with chronic autoimmune thyroiditis who had high titres of thyroid specific autoantibodies; in one out of 5 (20.0 %) whole blood DNA samples from patients with chronic thyroiditis who were within the normal range of TGAb, aTPOAb and/or TRAb autoantibodies. Besides, in one patient with high titre of thyroidspecific autoantibodies, HHV-6 genomic sequence was detected also in cell-free plasma DNA sample, which is a marker of active viral infection. HHV-6 genomic sequence was found also in 18 out of 28 (64.2 %) whole blood DNA samples from patients without chronic thyroiditis. HHV-6 genomic sequence in whole blood DNA samples was found in 8 out of 59 (13.5 %) healthy blood donors. HHV-6 was not found in any of the cell-free plasma DNA sample of patients without thyroiditis and control group individuals (Figure 2).
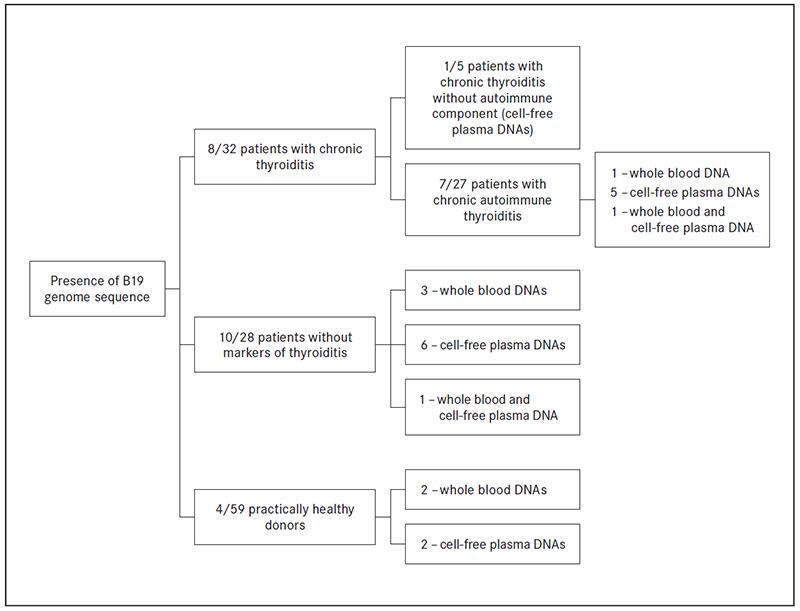
B19 genomic sequence was detected in 8 out of 32 (25.0 %) patients with chronic thyroiditis: in 7 out of 27 (one whole blood DNA; 5 cell-free plasma DNA; one whole blood and plasma DNA sample) patients with chronic autoimmune thyroiditis and in 1 out of 5 (one plasma DNA sample) patients with chronic thyroiditis without autoimmune component. DNA B19 genomic sequence was found also in 10 out of 28 (35.7 %) patients without chronic thyroiditis (3 whole blood DNA; 6 cell-free plasma DNA; one whole blood and plasma DNA sample) and 4 out of 59 (6.7 %) practically healthy donors (2 in whole blood DNA samples and 2 in cell-free plasma DNA samples (Figure 3).
Figure 1. The presence of TGAb, TPOAb and TRAb antibodies in patients with and without chronic thyroiditis and practically healthy blood donors
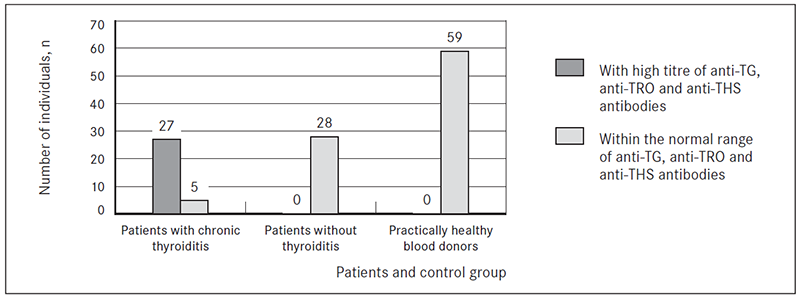
Figure 2. The presence of HHV-6 genome sequence in whole blood and cell-free plasma DNA samples from patients with and without chronic thyroiditis and practically healthy blood donors
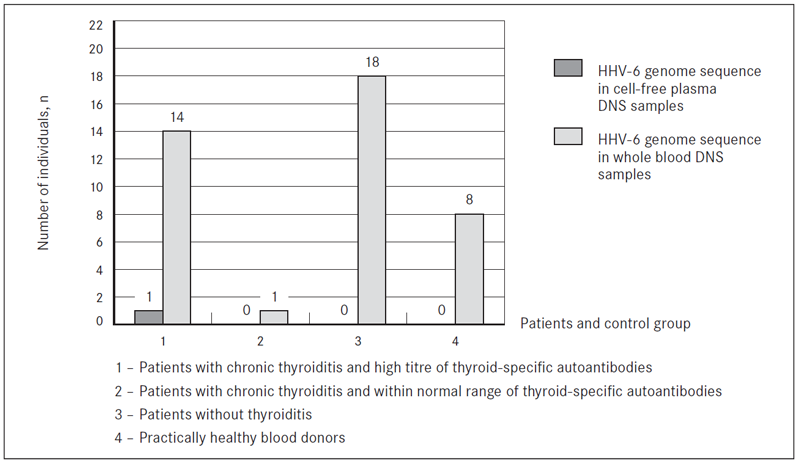
Figure 3. The presence of B19 genome sequence in DNA samples from patients with and without chronic thyroiditis and practically healthy blood donors
Discussion
Viral infections have been frequently cited as important environmental factor implicated in chronic autoimmune thyroiditis, but no specific virus infection has yet been associated to the disease. Despite long time since HHV-6 and B19 were discovered, these viruses offer continuous challenge to virologists.
We found that patients with chronic autoimmune thyroiditis are characterised by statistically significantly higher titres of thyroid-specific autoantibodies expression than practically healthy donors (p = 0.0001) confirming previously published data that from serological point of view autoantibodies against thyroid peroxidase, TSH receptor and thyroglobulin are the main markers of autoimmune thyroid gland diseases.
Nested polymerase chain reaction is an appropriate and sensitive method for the detection of viral markers in DNA samples, and it is the beginning of understanding the relationship between HHV-6 and/or B19 and thyroid gland diseases. HHV-6 was found in 55.5 % patients with chronic autoimmune thyroiditis which is statistically significantly more often than in practically healthy donors – 13.5 % (p = 0.0001). The presence of HHV-6 was also demonstrated in 20.0 % of patients with chronic thyroiditis without autoimmune component and 64.2 % of patients without chronic thyroiditis confirming Thomas and co-authors’ previously published data where they showed no differences in the incidence of HHV-6 infection in patients with or without thyroid autoimmune diseases [Thomas et al., 2008]. Very recently, experimental evidence supporting a role for HHV-6 in the aetiology of Hashimoto’s thyroiditis has been provided [Caselli, 2012]. The presence and transcriptional state of HHV-6 was detected in thyroid fine needle aspirates and peripheral blood mononuclear cells from patients with Hashimoto’s thyroiditis [Caselli, 2012].
Following primary infection, HHV-6 persists lifelong in the human host and viral reactivation is associated with a range of complications. However, much more research is needed to answer one of the most important issues – whether the virus causes clinical activity of the disease, or these viruses become activated due to pathological mechanism of the disease. It is very complex to evaluate the impact of each virus because many viruses are able to simultaneously exist in a body. For example, Chapenko and co-authors demonstrated that in renal transplant patients, human herpesvirus 7 is activated first, and only then HHV-6 [Chapenko et al., 2009], which could be explained by the fact that in case of HHV-7 infection CD46 and CD59 expression level increases in target cells [Takemoto et al., 2007]. In addition, there are studies, which show that other viruses have also been found in patients with thyroid gland diseases – Epstein-Barr virus Simian vacuolating virus 40, mumps virus and retroviruses [Kannangai, 2010; Desailloud, 2009].
Infection with B19 has been linked to a variety of diseases including erythroid, thyroid, neurological and autoimmune diseases. Lately, Ignatovich and Hobbs showed that the infection of primary CD36+ cells with B19 coincides with down-regulation of thyroid, retinoid, and oestrogen hormone receptors [Ignatovich, 2013]. Our results demonstrate that B19 genome sequence was found significantly more often in patients with chronic autoimmune thyroiditis – 25.9 % comparing with practically healthy donors – 6.7 % (p = 0.031). B19 was also found in one patient with chronic thyroiditis without autoimmune component and in 35.7 % patients without markers of chronic thyroiditis. Active B19 infection was observed in 6 patients with chronic thyroiditis (5 with chronic autoimmune thyroiditis and one patient with chronic thyroiditis without autoimmune component) and only in two practically healthy donors; however, the difference between patients and control group is not statistically significant (p = 0.159). It must not be ruled out that B19 is using cellular mechanisms, which are responsible for the immune response and therefore promoting the development of thyroid-specific autoantibodies.
Conclusion
HHV-6 and B19 infection was demonstrated in patients with chronic autoimmune thyroiditis significantly more often in comparison to practically healthy blood donors. At the same time, HHV-6 and B19 infection was also detected in patients with other thyroid gland pathologies, without statistical significance in virus infection frequency between patients with chronic autoimmune thyroiditis and other thyroid gland pathologies. To draw a conclusion on the involvement of HHV-6 and B19 infection in etiopathogenesis of chronic autoimmune thyroiditis resected thyroid tissues should be analysed for the presence of virus-specific DNAs and RNAs as well as for viral antigens expression.
Acknowledgment
This work was supported by the project 10.0029.4 “Beta-herpesviruses (HHV-6, HHV-7) and parvovirus B19 infections as possible risk factors for the development of autoimmune thyroid disease” and Taiwan-Latvia-Lithuania cooperation project “Establishing of framework to track molecular epidemiology of Parvoviruses and correlate sequence variability with different clinical manifestation”.
References
- Ablashi D. V., Salahuddin S. Z., Josephs S. F., et al. HBLV (or HHV-6) in human cell lines // Nature, 1987; 329 (6136): 207.
- Broccolo F., Drago F., Cassina G., et al. Selective reactivation of human herpesvirus 6 in patients with autoimmune connective tissue diseases // J Med Virol, 2013; 85 (11): 1925–1934.
- Chapenko S., Folkmane I., Ziedina I., et al. Association of HHV-6 and HHV-7 reactivation with the development of chronic allograft nephropathy // J Clin Virol, 2009; 46: 29–32.
- Caselli E., Zatelli M. C., Rizzo R., et al. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto’s thyroiditis // PLoS Pathog, 2012; 8 (10).
- Desailloud R., Hober D. Viruses and thyroiditis: An update // Virol J, 2009; 12: 6–15.
- Barah F., Chiswick M. L., Vallely P. J., et al. Association of human parvovirus B19 infection with acute meningoencephalitis // The Lancet, 2001; 358: 729–730.
- Kannangai R., Sachithanandham J., Kandathil A.J., et al. Immune responses to Epstein-Barr virus in individuals with systemic and organ specific autoimmune disorders // Indian J Med Microbiol, 2010; 28: 120–123.
- Mori K., Munakata Y., Saito T., et al. Intrathyroidal persistence of human parvovirus B19 DNA in a patient with Hashimoto’s thyroiditis // J Infect, 2007; 55: 29–31.
- Nora-Krukle Z., Chapenko S., Logina I., et al. Human herpesvirus 6 and 7 reactivation and disease activity in multiple sclerosis // Medicina (Kaunas), 2011; 47 (10): 527–531.
- Prummel M., Strieder T., Wiersinga W. M. The environment and autoimmune thyroid diseases // Eur J Endocrinol, 2004; 150: 605–618.
- Secchiero P., Carrigan D. R., Asano Y., et al. Detection of human herpesvirus 6 in plasma of children with primary infection and immunosuppressed patients by polymerase chain reaction // J Infect Dis, 1995; 171: 273–280.
- Takemoto M., Yamanishi K., Mori Y. Human herpesvirus 7 infection increases the expression levels of CD46 and CD59 in target cells // J Gen Virol, 2007; 88: 1415–1422.
- Thomas D., Liakos V., Michou V., et al. Detection of herpes virus DNA in post-operative thyroid tissue specimens of patients with autoimmune thyroid disease // Exp Clin Endocrinol Diabetes, 2008; 116: 35–39.
- Vandamme A. M., Fransen K., Debaisieux L., et al. Standardisation of primers and an algorithm for HIV-1 diagnostic PCR evaluated in patients harbouring strains of diverse // J Virol Methods, 1995; 51 (2–3): 305–316.
- Vejlgaard T. B., Nielsen O. B. Subacute thyroiditis in Parvovirus B19 infection // Ugeskr Laeger, 1994; 156 (41): 6039–6040.
- Wang J., Zhang W., Liu H., et al. Parvovirus B19 infection associated with Hashimoto’s thyroiditis in adults // J Infect, 2010; 60 (5): 360–370.



