Comparative Study of Overall Survival of Hereditary (HNPCC) and Other Forms of Colorectal Cancer according to Family History
Abstract
Cancer is a very common disease; many families have at least a few members who have had cancer. Sometimes, certain types of cancer affect some families. This can be caused by a number of factors but in some cases cancer is caused by mutation and determinated genetically.
The purpose of this study was to describe compatibility to Amsterdam criteria II (ACII) of various substitute classification systems by comparing overall survival of hereditary non-polyposis colorectal cancer (HNPCC) patients and colorectal (CRC) patients with suspicion to HNPCC syndrome in family; patients with cancer aggregation among more than 3 first-degree relatives in family; and other patient groups with hereditary cancer of localizations related to Lynch syndrome.
The case-record data of 1423 patients were analyzed, classified by clinical diagnosis, cancer history in family, age at which cancer was diagnosed, stages of cancer by TNM classification, criteria matching and mortality.
Patients with CRC diagnosis without known cancer cases of any localization among first and seconddegree relatives in family have worst survival rate compared to patients in HNPCC group matching to Amsterdam criteria II. Comparing the survival between patients with sporadic and hereditary colorectal cancer, survival rate as prognostic factor among different patients groups with or without cancer diagnosis (CRC, breast or other types), anamnesis in family comparing with HNPCC syndrome the study reported improved prognosis for HNPCC patients compared to sporadic colorectal cancer patients.
Probably survival of HNPCC patients depends not only on family history, tumour localization, pathohistological findings, but also on biological and genetic features.
In this context, molecular genetic testing of MMR gene mutations would play a key prognostic role.
Introduction
Cancer is such a common disease that many families have at least a few members who have had cancer. Sometimes, certain types of cancer seem to run in some families. This can be caused by a number of factors, like smoking, unhealthy life style, obesity that tend to influence cancer risk. In some cases cancer is caused by mutation that is being passed over from generation to generation. Although this is often referred to as an inherited cancer, what is inherited is the mutation that can lead to cancer, not the cancer itself. Only about 5‒10 % of all cancers are hereditary – resulting directly from mutation inherited from a parent [2].
One cause of hereditary colon cancer is a disease called familial adenomatous polyposis (FAP). People with this disease start getting colon polyps in adolescence, and over time may have many of polyps in their colon. If left alone, at least one of these polyps will become cancer. The gene for this syndrome is called APC, and testing for mutations in this gene is available. If FAP is diagnosed early in life, surgery to remove the colon is often used to stop the cancer from developing. The most common inherited syndrome that increases a person’s risk for colon cancer is hereditary non-polyposis colorectal cancer (HNPCC), or Lynch syndrome. People with this syndrome have a high risk of colorectal cancer. Most of these cancers occur before age 50. HNPCC also leads to a high risk of endometrial cancer in women. Other cancers linked with HNPCC include cancer of the ovary, stomach, small intestine, pancreas, kidney, brain, urethras and bile duct [1].
HNPCC is caused by mutations in one of DNA mismatch repair (MMR) genes – MLH1, MSH2, MSH6, PMS1, or PMS2. Mutations in these genes can be found by genetic testing. Another option for people with colorectal cancer is to have the tumour tissue tested for changes that can be caused when one of these genes is faulty. These changes are known as microsatellite instability (MSI). Having normal findings (no MSI) implies that HNPCC is not present and that the genes that cause it are normal [10.] Synchronous and metachronous cancers are common, and multiple colorectal cancers can be observed in 20–40 % of people with HNPCC [12].
The ones who are known to carry an MMR gene mutation should start colonoscopy screening at a young age in order to diagnose cancers and polyps at an early stage. Women with HNPCC should be screened for endometrial cancer.
Originally, HNPCC is a clinical diagnosis, based on the Amsterdam criteria I (ACI). In 1999, the ACI were superseded by the Amsterdam criteria II (ACII), which included extracolonic HNPCC-associated tumours. After the discovery of germline MMR gene mutations as the cause of HNPCC, it soon became challenged that these criteria are too stringent, as a large proportion of families carrying germline MMR gene mutations did not fulfill either ACI or ACII criteria. Due to cultural and socio-economic reasons and the size of Latvian families, in clinical practice it is often hard to fulfill ACII criteria and get an appropriate anamnesis for patients diagnosed with CRC. Consequently, several groups formulated new sets of criteria to select patients for mutation analysis in the MMR genes. The best known are the Bethesda criteria, published in 1997 and revised in 2004 and HNPCCsusp. groups by Józef Kładny and Jan Lubiński [8].
The aim
The aim of this study is to describe usefulness and compatibility to ACII of various substitute classification systems by comparing overall survival of HNPCC patients and CRC patients classified as HNPCCsusp. (suspicion to HNPCC syndrome in family); patients with cancer aggregation among more than 3 first-degree relatives in family (group CFA); and other patient groups with hereditary cancer of localizations related to Lynch syndrome.
The results should enable to give recommendations to rational guidelines regarding whether or not to offer mutation analysis to a patient with cancer who is at risk of HNPCC and could be used as prognostic marker in clinical practice.
Material and methods
The case-record data of 1423 patients at mean age 67.1 years were obtained from Rīga Stradiņš University Institute of Oncology data base. Patients were classified by clinical diagnosis, cancer history in family, age at which cancer was diagnosed, stages of cancer by TNM classification, criteria matching. The mortality was analyzed using the Cox proportional hazards models. The diagnosed group, age, and stratified tumour stages were tested with multivariable linear regression models adjusted for covariates. Methodology and criteria by Józef Kładny and Jan Lubiński were used to divide patients into HNPCC and HNPCCsusp. groups [8].
The R software version 2.15.1 was used for the statistical analyses. All hypotheses were two sided and p-value less than 0.05 was considered as statistically significant.
Results
Patient groups. 114 (8.6 %) patients were diagnosed stage I cancer, 501 (37.7 %) – stage II, 424 (31.9 %) patients – stage III, 289 (21.8 %) patients – stage IV cancer by TNM classification [6]. For 113 patients cancer stage data were not available. We analyzed 356 (25 %) patients with 2 cases of any cancer localization in family (2CA group); 144 (10.1 %) patients with more than 3 first-degree relatives with any cancer diagnosis (CFA group); 85 (6 %) patients with 2 cases of CRC within I0 blood relatives older than 50 years (FCC1 group ‒ Family Colorectal Cancer); 30 (2.1 %) patients with 2 cases of CRC within II0 blood relatives at any age (FCC2 group); 16 (1.1 %) patients with hereditary breast cancer in family (HBC group) – at least 3 breast cancer cases in one family at any age, one of them a firstdegree relative to other two; 47 (3.3 %) patients matching to Amsterdam criteria II; 76 (5.3 %) patients (HNPCCsusp. group – suspicion to HNPCC syndrome in family) – at least 2 first-degree relatives had HNPCC syndrome related cancer, at least one out of them diagnosed before age 50; 669 (47 %) CRC patients as a control group without any detected hereditary cancer or known cancer anamnesis among first or second-degree relatives in family, defined as NEGATIVE group (Table 1).
Mean survival of all analyzed patients were 48.2 months.
Statistical analysis. Survival of different patients groups graphically shows Kaplan-Meijer survival curves. Vertical axis represents estimated probability of survival for a hypothetical cohort (survival rate, the percentage of subjects who have survived), horizontal axis represents living for a certain amount of time after treatment, measured in months.
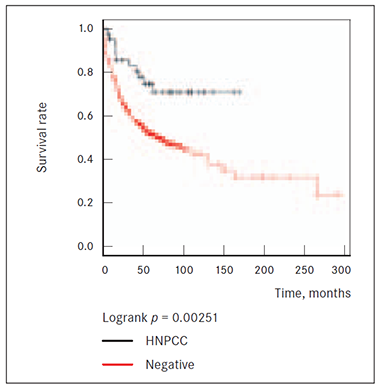
It appears that patients with CRC diagnosis without known cancer cases of any localization among first and second-degree relatives in family (NEGATIVE group) have worst survival rate compared to patients in HNPCC group matching to Amsterdam criteria II. Cox multivariate analysis showed that the hazard ratio of risk of death from cancer was 0.4 (95 % CI 0.2‒0.8; p = 0.01) in HNPCC (Figure 1).
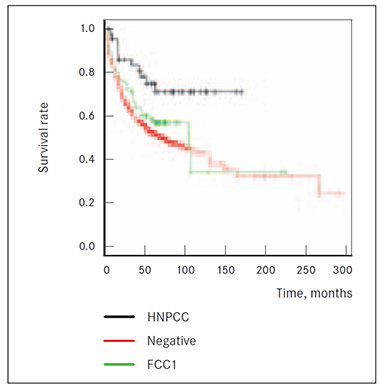
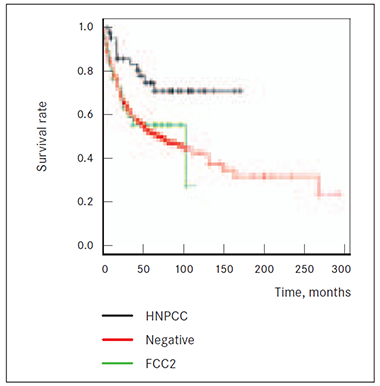
Also 2 CRC cases among first or second-degree blood relatives in anamnesis do show significantly (p = 0.0315 and p = 0.0367, respectively) worst survival prognosis than HNPCC group patients and death risk from cancer is higher in both FCC1 and FCC2 than in HNPCC group (Figures 2, 3). Results did not reach statistical significance that patients of groups FCC1 and FCC2CRC (at least 2 cases of CRC cases among first and second degree relatives (p = 0.261 and p = 0.784)) would have higher death risk from cancer than patients without known cancer anamnesis in family (NEGATIVE group) (Figures 2, 3).
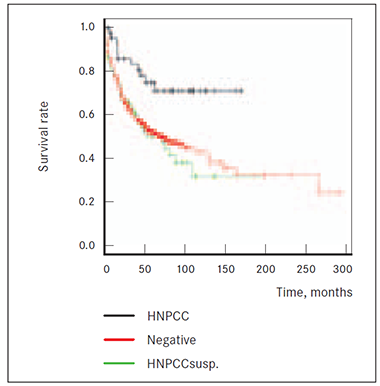
Analysis also showed lower potential risk to die from cancer being diagnosed with HNPCC by Amsterdam criteria II than for patients (HNPCCsusp. group) with first-degree relatives who have had HNPCC syndrome related cancers (colonic, or extra-colonic) (p = 0.0021). However, sporadic CRC cannot be considered as a better prognostic factor for disease outcome compared to patients’ group with anamnesis of at least 2 relatives having HNPCC syndrome related cancers and one of them diagnosed before age 50 (p = 0.784) (Figure 4).
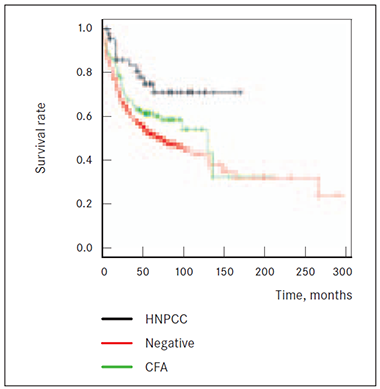
Patients group matching to Amsterdam criteria II (HNPCC) did show better survival prognosis than group with any type of cancer diagnosis aggregation within family among the first-degree relatives (CFA group) (p = 0.049). Comparing CFA group with patients without known cancer anamnesis in family (NEGATIVE group) median survival difference is 5.2 months; however there is no statistical significance reached proving risk for death within 14‒15 years (p = 0.060) (Figure 5).
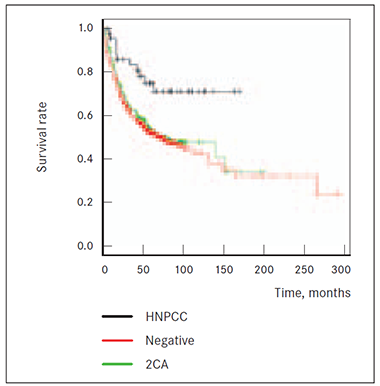
Also 2 CRC cases among first or second-degree relatives in anamnesis (2CA group) showed significantly (p = 0.007) worst survival prognosis than HNPCC group patients, but death risk from cancer is not higher (p = 0.444) compared to patients without relatives having had any known cancer diagnosis (NEGATIVE group) (Figure 6.)
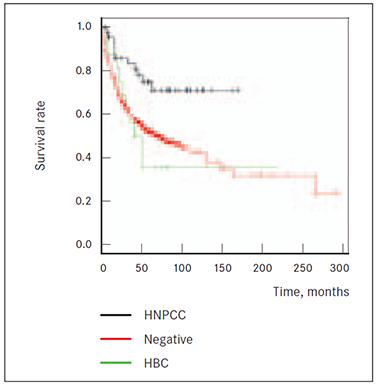
Although patients’ group having had hereditary breast cancer cases in family (HBC group) showed worst survival prognosis compared to HNPCC group (p = 0.0106) and median survival difference is 12.4 months, there is no proven survival benefit of NEGATIVE group over HBC group (p = 0.639) (Figure 7).
Table 1. Groups of patients, diagnosis and clinical data analyzed, spread by cancer stages*
| Group of patients | Clinical description | Number of patients, n | Age median, years (IQR) | Survival median, months (IQR) | Stage of cancer – number of patients in the group, n (%) |
|---|---|---|---|---|---|
| 2CA | Two cases of any cancer localization in consanguineous patients | 356 | 68 (60, 75) | 46.2 (17.2, 69.7) | I – 30 (9.0 %) |
| II – 132 (39.6 %) | |||||
| III – 103 (30.9 %) | |||||
| IV – 68 (20.4 %) | |||||
| 23 – stage data unavailable | |||||
| CFA | Cancer familial aggregation: ≥ 3 diagnosis of cancer among first-degree relatives | 144 | 68.5 (59, 76) | 50.5 (22.3, 74.5) | I – 15 (10.9 %) |
| II – 58 (42.3 %) | |||||
| III – 40 (29.2 %) | |||||
| IV – 24 (17.5 %) | |||||
| 7 – stage data unavailable | |||||
| FCC1 | Family colorectal cancer 1: 2 CRC cases among first-degree-relative patients with age-of-onset ≥ 50 years | 85 | 68 (62, 75) | 51.1 (24.9, 70.5) | I – 7 (8.9 %) |
| II – 32 (40.5 %) | |||||
| III – 25 (31.6 %) | |||||
| IV – 15 (19.0 %) | |||||
| 6 – stage data unavailable | |||||
| FCC2 | Family colorectal cancer 2: 2 CRC cases among second-degree-relative patients with any age-of-onset | 30 | 62 (53.2, 71.5) | 51.9 (16.7, 77.2) | I – 5 (17.9 %) |
| II – 9 (32.1 %) | |||||
| III – 8 (28.6 %) | |||||
| IV – 6 (21.4 %) | |||||
| 2 – stage data unavailable | |||||
| HBC | Hereditary breast cancer: ≥ 3 breast cancer cases in family with any age-of-onset; one first-degree relative to other two diagnosed cases | 16 | 62.5 (57.8, 72.5) | 44.7 (25.4, 68.5) | I – 0 (0.0 %) |
| II – 4 (26.7 %) | |||||
| III – 7 (46.7 %) | |||||
| IV – 4 (26.7 %) | |||||
| 1 – stage data unavailable | |||||
| HNPCC | Amsterdam’s criteria II: 1) ≥ 3 relatives diagnosed with HNPCC or related cancer (colorectal, endometrial, small intestine, renal, urethral cancer), one first-degree relative to other two diagnosed cases; 2) diagnosed cancer in two generations; 3) ≥ 1 case of cancer before age of 50; 4) diagnosis of FAP should be excluded | 47 | 66 (57.5, 73) | 57.1 (20.9, 88.8) | I – 5 (11.6 %) |
| II – 16 (37.2 %) | |||||
| III – 17 (39.5 %) | |||||
| IV – 5 (11.6 %) | |||||
| 4 – stage data unavailable | |||||
| HNPCC susp. | HNPCC syndrome in family suspected: ≥ 2 cases of HNPCC syndrome related cancer in first-degree relatives with one case diagnosed before age of 50 | 76 | 60 (48.8, 69) | 35.4 (16.4, 69.9) | I – 5 (7.4 %) |
| II – 25 (36.8 %) | |||||
| III – 22 (32.4 %) | |||||
| IV – 16 (23.5 %) | |||||
| 8 – stage data unavailable | |||||
| Negative | Control group: diagnose of CRC without hereditary cancer or known cancer anamnesis in family | 669 | 70 (63, 76) | 45.3 (15.5, 68.4) | I – 47 (7.5 %) |
| II – 225 (36.0 %) | |||||
| III – 202 (32.3 %) | |||||
| IV – 151 (24.2 %) | |||||
| 44 – stage data unavailable |
* Stage of cancer by TNM classification.
Figure 1. Survival of HNPCC and NEGATIVE patients groups | Figure 2. Survival of HNPCC, NEGATIVE and FCC1 groups |
|
|
Figure 3. Survival of HNPCC, NEGATIVE and FCC2 groups | Figure 4. Survival of HNPCC, NEGATIVE and HNPCCsusp. groups |
|
|
Figure 5. Survival of HNPCC, NEGATIVE and CFA groups | Figure 6. Survival of HNPCC, NEGATIVE and 2CA groups |
|
|
Figure 7. Survival of HNPCC, NEGATIVE and HBC groups | |
|
Discussion
Different survival rates of patients with colorectal cancer have been investigated in several studies.
The results are sometimes conflicting because of the different pathogenetic mechanism of tumour genesis between sporadic and familiar types of colorectal syndrome (HNPCC in particular). These differences are probably due to different clinical pathological characteristics and genetic alterations [4].
If we compare the survival between patients with sporadic and hereditary colorectal cancer, survival rate as prognostic factor among different patients groups with or without cancer diagnosis (CRC, breast or other types) anamnesis in family compared to HNPCC syndrome, then a couple of studies have reported improved prognosis for HNPCC patients compared to sporadic colorectal cancer patients, but some studies did not confirm it [15].
The localization of tumour is an important prognostic factor for survival [4], because some types of cancer are more aggressive than the other ones. Moreover, the same colorectal cancer based on anatomical localization (rectum, colon), distribution and histological characteristic of tumour can influence survival prognosis.
This different anatomical distribution between HNPCC and sporadic CRC, confirmed in literature [5] is one of the Amsterdam criteria for the diagnosis of HNPCC and determines a better prognosis, being less aggressive.
We have considered survival rate, stratified patients by groups based on cancer anamnesis in family among first and second degree relatives – whether they had CRC or any other cancer localization, were diagnosed at an early age (< 50 years) or by criteria suspected to HNPCC syndrome.
5-year survival in HNPCC group was significantly better than in sporadic CRC group, it means less death risk from cancer (95 % CI 0.2‒0.8; p = 0.01) in HNPCC.
Survival rate of HNPCC showed also benefit versus patients with any type of cancer aggregation within family (2CA and CFA groups), also previous history of 2 cases of CRC diagnosis among first and second-degree relatives could be considered as poorer prognostic factor for survival than HNPCC syndrome.
Interestingly, even patients group suspected to HNPCC syndrome (HNPCCsusp.) showed much worst survival prognosis than by Amsterdam criteria defined HNPCC group (p = 0.002).
Hereditary breast cancer patient group (HBC) with at least 3 breast cancer cases in family showed much worst survival prognosis than HNPCC group (p = 0.01).
Although patients in each group were spread statistically similarly taking into account cancer stage by TNM classification (Table 1), prevalence of localized tumours (stage II and stage III) in HNPCC group is higher than in HNPCCsusp. group where more advanced disease (stage IV for 16 patients out of 76 vs. 5 patients out of 47) is found more often. Current analysis did not compare different patient groups by different cancer stages; this could be valuable information for further research based on some studies [9, 5] indicating that a good prognosis can be based on a favourable stage at diagnosis.
When matched stage for stage, colon cancers in individuals with Lynch syndrome are associated with a better prognosis than sporadic colon cancers. Also, poorly differentiated histology of Lynch syndrome-related colon cancers is typically associated with a poor prognosis. Histologic characteristics of Lynch syndrome-related colon cancers include: poor differentiation, tumour-infiltrating lymphocytes, mucin, and signet ring or cribiform histology [14]. Histological findings were not taken into account at this study.
Further studies should deal with different therapies patients of different groups received – whether adjuvant chemotherapy was received or not, what was the regimen, the number of chemotherapy cycles.
Lynch syndrome is caused by mutations in genes involved with MMR pathway, and this pathway functions identify and remove single nucleotide mismatches or insertions and deletion loops. Mutations in four of the MMR genes can cause Lynch syndrome [14]. The functions of the mismatch repair genes can be disrupted by missense mutations, truncating mutations, splice site mutations, large deletions, or genomic rearrangements. In addition, germline deletion within EPCAM, which is not an MMR, can disrupt the MMR pathway. Genetic modifiers of cancer risk in Lynch syndrome have been reported by many authors [14]. To establish the probability of Lynch syndrome and to identify which gene is most likely to have a causative germline mutation in a person with Lynch syndrome, tumour testing is recommended.
Although the Amsterdam criteria can be a significant predictor of a germline mutation in an MMR gene in families that fulfill the criteria, the Amsterdam criteria, nonetheless, fail to identify a large portion of persons with a germline MMR gene mutation. Therefore, family history and the Amsterdam criteria cannot be relied upon to identify all individuals with a germline mutation in one of the MMR genes. Sjursen et al. [2010] found the sensitivity of the Amsterdam criteria II to be 87 %, 62 %, 38 %, and 48 % for identifying persons with a MLH1, MSH2, PMS2, or MSH6 germline mutation, respectively [14]. Hampel et al. also reported that in a population-based study of persons with colon cancer, only three of 23 persons with a germline mutation in an MMR gene met the Amsterdam criteria [6].
Therefore besides cancer anamnesis in family, assessment matching to Amsterdam criteria, life style evaluation and clinical assessment of molecular genetic testing is recommended.
There are reported advantages of Microsatellites Instabity (MSI) testing. MSI testing is an effective method for determining which tumours arise from MMR deficiency. Studies have demonstrated that the sensitivity of MSI testing for identifying tumours in individuals with a germline MMR gene mutation is 93 % [16].
BRAF mutations the most commonly occur in 15 % of colorectal cancers. BRAF mutations are thought to be rare in Lynch syndrome-related cancers and, thus, in general the presence of a BRAF mutation rules out the diagnosis of Lynch syndrome [14].
There are many Colorectal Cancer Screening testing strategy guidelines developed by professional associations in Europe and the USA, e.g., the National Comprehensive Cancer Network (NCCN) [11].
The screening of tumour tissue for MSI allow to identify individuals who may have Lynch syndrome.
Predictive testing for at-risk asymptomatic adult family members requires prior identification of the disease-causing mutation in the family. Penetrance of colon cancer associated with mutations in a MMR gene or EPCAM is less than 100 %. Therefore, some individuals with a cancer-predisposing mutation in one of the MMR genes never develop colon cancer [14].
Clinicians and researchers working in the area of hereditary colon cancer have suggested returning to the Lynch syndrome instead of HNPCC in order to specify individuals and families with defective MMR and to distinguish them from other forms of familial colon cancer [3].
Colon cancer surveillance should be ensured by regular colonoscopy, removal of precancerous polyps reduces the incidence of colon cancer in individuals with Lynch syndrome. A 2009 study of a Finish cohort with high compliance with screening found no increase in mortality for individuals with Lynch syndrome over their mutation-negative relatives, indicating that annual colonoscopy could help with the prevention and detection of colon cancer [7]. Therefore, current recommendations are to have colonoscopy every one to two years beginning between ages 20 and 25 years or ten years before the earliest diagnosis in the family, whichever is earlier [7].
Some authors also report that cigarette smoking increases the risk of colorectal cancer in Lynch syndrome [13].
Early recognition of cancers associated with Lynch syndrome may allow for timely intervention and improved final outcome.
When an MMR gene mutation has been identified in a family with Lynch syndrome, molecular genetic testing for the mutation should be offered to all first-degree relatives.
Several factors can hinder the diagnosis of Lynch syndrome based on family history. Screening and removal of precancerous polyps and prophylactic surgery may prevent colon or endometrial cancer in some at-risk relatives; some who died young from other causes may never have developed cancer [14].
However genetic cancer risk assessment and testing is not useful in predicting whether symptoms will occur, and if they do, what the age of onset, severity and type of symptoms, or rate of disease progression will be. When testing at-risk individuals for Lynch syndrome, an affected family member should be tested first to confirm the molecular diagnosis in the family.
Conclusions
- Our findings appear to confirm previous studies which detected that a better survival for colon cancer in HNPCC, compared to sporadic CRC, usually occur. Moreover, HNPCC patient has better prognostic survival prognosis than hereditary breast cancer or CRC or other localization cancer diagnosis in family (within first and second-degree relatives).
- Probably survival of HNPCC patients depends not only on family history, tumour localization, pathohistological findings, but also on biological and genetic features.
- In this context, molecular genetic testing of MMR gene mutations would play key prognostic role. MSI mutation pattern plays an important prognostic role since colon cancer with MSI has a better prognosis than tumours without MSI.
- HNPCC group should be enlarged and divided by stages, clinical features, tumour histological type and dispensed therapy, and be examined to confirm this data. Only family cancer history and compatibility to ACII criteria with various substitute classification systems is an insufficient prognostic marker for survival in clinical practice.
- Research should be widened to give recommendations to rational guidelines of molecular genetic analysis and result interpretation to a patient with cancer who is at risk of HNPCC based on clinical, pathological and genetic findings.
References
- American Cancer Society Cancer Information Database. Colorectal Cancer // http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedG… (viewed 2013.05.11).
- American Cancer Society Cancer Information Database. Heredity and Cancer // http://www.cancer.org/cancer/cancercauses/geneticsandcancer… (viewed 2013.05.11).
- Bellizzi A. M., Frankel W. L. Colorectal cancer due to deficiency in DNA mismatch repair function: a review // Adv Anat Pathol, 2009; 16: 405–417.
- Cosimelli M., Stigliano V., Assisi D., et al. Survival of hereditary non-polyposis colorectal cancer patients compared with sporadic colorectal cancer patients // Journal of Experimental & Clinical Cancer Research, 2008; 27: 39.
- Frattini M., Balestra D., Suardi S., et al. Different genetic features associated with colon and rectal carcinogenesis // Clin Cancer Res, 2004 Jun 15; 10 (12 Pt 1): 4015‒4021.
- Hampel H., Stephens J. A., Pukkala E., et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset // Gastroenterology, 2005; 129: 415–421.
- Järvinen H. J., Renkonen-Sinisalo L., Aktán-Collán K., et al. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation negative family members // J Clin Oncol, 2009; 27: 4793–4797.
- Kadny J., Lubiński J. Hereditary Lynch syndrome (HNPCC) // Cancer in Clinical Practice, 2008; 6 (2): 99‒102.
- Kohlmann W., Gruber M. S., Stephen B., MD, PhD. Lynch Syndrome synonyms: HNPCC, Hereditary Non-polyposis Colon Cancer. Includes: EPCAM-related Lynch Syndrome, MLH1-related Lynch Syndrome, MSH2-related Lynch Syndrome, MSH6-related Lynch Syndrome, PMS2-related Lynch Syndrome // http://www.ncbi.nlm.nih.gov/books/NBK1211/#hnpcc.Molecular_… / Initial posting: February 5, 2004; last revision: September 20, 2012 (viewed 2013.04.24).
- National Human Genome Research Institute. Chromosome abnormalities fact sheet // www.genome.gov/11508982 on December 14, 2011 (viewed 2013.04.20).
- NCCN Clinical Practice Guidelines in Oncology: Colon Cancer // http://www.nccn.org/professionals/physician_gls/pdf/colon.p… (viewed 2013.04.20).
- Niessen R. C., Berends M. J., Wu Y., et al. Identification of mismatch repair gene mutations in young patients with colorectal cancer and in patients with multiple tumours associated with hereditary non-polyposis colorectal cancer // Gut, 2006; 55: 1781–1788; doi: 10.1136/gut.2005.090159.
- Pande M., Lynch P. M., Hopper J. L., et al. Smoking and colorectal cancer in Lynch syndrome: results from the Colon Cancer Family Registry and the University of Texas M. D. Anderson Cancer Center // Clin Cancer Res, 2010; 16: 1331–1339.
- Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer // J Clin Oncol, 2003; 21: 1174–1179.
- Percesepe A., Benatti P., Roncucci L., et al. Survival analysis in families affected by hereditary nonpolyposis colorectal cancer // Int J Cancer, 1997; 71: 373‒376.
- Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part 1: The utility of immunohistochemistry // J Mol Diagn, 2008; 10: 293–300.