Detection of CDH1 Gene Variants in Patients with Hereditary Diffuse Gastric Cancer by Denaturing High Performance Liquid Chromatography
Abstract
Germline variants in the CDH1 gene are the major cause of hereditary diffuse gastric cancer (HDGC) and are identified in approximately 25–40 % of families which fulfil strict criteria. Because these variants are spread over the entire gene, their detection requires sequencing of all 16 exons. For screening sequence variations in genes, rapid turnover time is of fundamental importance. Many of the current methods are time consuming and technically difficult to implement. Denaturing high performance liquid chromatography (DHPLC) has been shown to be a highly sensitive, time saving and economical method for variant screening.
In the present study DHPLC method was used to screen patients who fulfilled the hereditary gastric cancer criteria for variant in CDH1 to confirm diagnosis.
DNA from 20 patients was screened for variant in the 16 exons of the CDH1 gene, using DHPLC and approved using direct sequencing.
From 20 patients’ families, three had at least two gastric cancer cases with one case of gastric cancer in a person younger than 50 years; 10 had multiple cases of gastric cancer diagnosed in person older than 50 years and seven patients were younger than 40 years. All gastric cancer patients were with poorly differentiated adenocarcinoma. DHPLC evaluation of the CDH1 gene revealed sequence polymorphism (c.1937-13T>C, rs2276330) without clinical significance in 5 patients. No variation was identified in nine patients. Variant c.*54C>T was identified in six patients.
We have demonstrated the feasibility of DHPLC analysis as a sensitive and rapid method for the analysis of the CDH1 gene in patients with hereditary diffuse gastric cancer.
Introduction
The E-cadherin is a member of the transmembrane glycoprotein family responsible for calciumdependent, cell-to-cell adhesion and plays a fundamental role in maintenance of cell differentiation and normal architecture of epithelial tissues [1, 509; 3, 74; 5, 6; 17, 1]. The protein is encoded by the CDH1 gene which is located on chromosome 16q22.1 and consists of 16 exons. Variants in CDH1 gene are known to be associated with Hereditary Diffuse Gastric Cancer syndrome (HDGC) [4, 1; 6, 436; 7, 4086; 8, 1705].
The HDGC has been defined in 1999 by International Gastric Cancer Linkage Consortium (IGCLC) as two or more documented cases of diffuse gastric cancer (DGC) in first or second degree relatives, including at least one case of DGC diagnosed before the age of 50, three or more documented cases of DGC in first or second degree relatives diagnosed at any age, isolated individual diagnosed with DGC at less than 45 years of age, isolated individual diagnosed with both DGC and lobular breast cancer [9, 250; 10, 1782; 12, 2360].
Among patients that fulfil the above clinical criteria about 25–40 % of cases carry a pathogenic variant in the CDH1 gene [14, 364; 18, 2137; 19, 646]. Patients with germline variants in the CDH1 have a high risk of developing diffuse gastric cancer and female carriers are at high risk of lobular breast cancer [20, 1349].
Analysis of CDH1 gene variants is important in patients fulfilling the HDGC criteria. Techniques for variant detection in disease related genes need to be sensitive and specific. Therefore the ideal method to use for variant analysis should be sensitive, non-hazardous, relatively inexpensive, and semi or fully automated to minimise labour and laboratory costs.
Denaturing high-performance liquid chromatography (DHPLC) had been shown to meet these criteria for variant screening. Denaturing high pressure liquid chromatography (DHPLC) is a relatively new technique, which uses heteroduplex formation between wild-type and mutated DNA strands to identify variants. Heteroduplex molecules are separated from homoduplex molecules by ion-pair, reversephase liquid chromatography on a special column matrix with partial heat denaturation of the DNA strands [13, 1735; 21, 336; 22, 956].
DHPLC is potentially a very useful method for the screening of a large number of samples for variants. Numerous reports during the last few years have documented the high accuracy and excellent sensitivity of DHPLC (96–100 %) in detecting variants in more than 250 genes [13, 1737]. DHPLC appears to be a reliable method specifically for the analysis of large genes known to be highly polymorphic and with a large variety of pathogenic variants [13, 1737; 22, 961].
In the present study DHPLC method was used to screen patients who fulfilled the hereditary gastric cancer criteria for variants in CDH1.
Material and Methods
Patients. Twenty patients from families fulfilling the criteria of HDGC according to IGCLC guidelines were selected for analysis of CDH1 gene. Three patients had at least two gastric cancer cases with one case of gastric cancer in a person younger than 50 years; ten had multiple cases of gastric cancer diagnosed in person older than 50 years and seven patients were younger than 40 years. All gastric cancer patients were with poorly differentiated adenocarcinoma.
PRC amplification conditions for DHPLC analysis of the CDH1 gene. Samples used for variant screening were amplified with PCR, using Optimase polymerase (Transgenomic) according to manufacturer’s guidelines. Primers for CDH1 all 16 exons were as reported by Mullins et al. [16, 753]. Reference sequence for CDH1 is NM 004360.3.
DHPLC analysis. All samples after PCR were denatured at 95 °C and gradually cooled to 25 °C to promote heteroduplex formation. DHPLC analysis was carried out on Transgenomic Wave 4500 HT, system (Transgenomic) using partially denaturing conditions, using temperature and flow gradient as suggested by the system for each amplicon.
Fragments showing an abnormal DHPLC pattern were investigated for identification of sequence variants by direct sequencing using ABI3130 and SeqScape program (Applied Biosystems).
Results
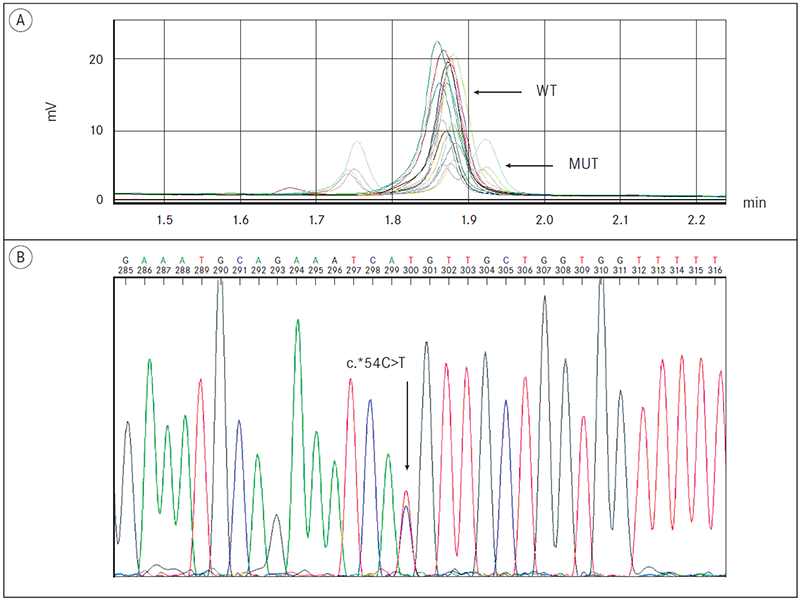
The 16 exons of CDH1 were screened for variant using DHPLC in 20 patients with hereditary gastric cancer. DHPLC evaluation revealed one sequence variation in six patients. They all had double peak in DHPLC chromatograms for exon 16 indicating heteroduplex formations (Figure 1a). Direct sequencing revealed c.*54C>T variant rs1801026 in all six patients (Figure 1b). Five patients had sequence polymorphism (c.1937-13T>C, rs2276330) without clinical significance. No genetic changes in CDH1 were identified in nine patients.
Figure 1. DHPLC detection of E-cadherin germline mutations and confirmatory direct sequencing analysis. DHPLC and corresponding sequence chromatograms of fragments of the E-cadherin gene:
(A) The amplicons containing exon 16, showed a different elution profile compared to the wild type samples;
(B) Confirmatory direct sequencing of a new PCR-product identified the variant c.*54C>T
Discussion
CDH1 germline variants are associated with the development of the autosomal cancer syndrome namely Hereditary Diffuse Gastric Cancer (HDGC) [1, 508; 2, 874; 4, 1; 9, 250]. About 25–40 % of families fulfilling the clinical criteria for HDGC established by the International Gastric Cancer Linkage Consortium (IGCLC) have constitutional alterations of the CDH1 gene. The offspring of an affected individual has a 50 % risk of also being affected.
The estimated cumulative risk of gastric cancer by the age of 80 is 67 % for men and 83 % for women. Women also have a 39 % risk for lobular breast cancer [14, 364; 18, 2137]. The penetrance of CDH1 gene variants is high. The lifetime risks of diffuse gastric cancer is 80 % in both men and women by the age of 80, and lobular breast cancer is 60 % in women by the age of 80.
Germline variants in CDH1 gene were originally reported in three Maori families with aggregation of diffuse gastric cancer [1, 515; 2, 874; 9, 250; 19, 649]. Since this report, several studies have investigated the role of CDH1 variants in gastric cancer in different ethnic groups [4, 2; 19, 649]. Different patterns of CDH1 germline variants have been described as truncating, deletion, insertion, splice site, nonsense, silence, and at last, missense alterations [9, 253].
Genetic testing should be initiated in affected patients. Many different methods have been used for identifying CDH1 variants. As gold standard for variant detection has been direct sequence analysis. However, the cost and effort of DNA sequencing is often considerable, especially in genetically heterogeneous diseases [21, 335]. In our study we applied DHPLC analysis to the detection of sequence variants in the CDH1 gene. DHPLC provides information about whether a variant is present.
In order to determine the specific nature of the variant, however, PCR products in question need to be sequenced. DHPLC evaluation of the CDH1 gene revealed sequence polymorphism (c.1937-13T>C, rs2276330) without clinical significance in 5 patients. Variant c.*54C>T was identified in six patients. Some clinical studies have demonstrated an association of variant c.*54C>T with tumour progression in HDGC, although the results are controversial [11, 993; 15, 861; 23, 26]. No variation was identified in nine patients.
Variant detection by DHPLC, as it is presented in our study, is a high-throughput, time saving, and economical tool for variant screening.
Conclusion
The presented study demonstrates the feasibility of DHPLC analysis as a sensitive and rapid method for the examination of the CDH1 gene in patients with hereditary gastric cancer. DHPLC may be cost-effective in the diagnosis of this disorder compared with other analytical methods as Single-strand conformation polymorphism (SSCP) and Denaturing gradient gel electrophoresis (DGGE) and a valuable alternative to the direct sequencing of all 16 exons of the CDH1 gene.
Acknowledgments
We gratefully acknowledge the support from the National research programme “Biomedicine for Public Health”, 2014–2017.
References
- Brooks-Wilson A. R., Kaurah P., Suriano G., et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet, 2004; 41 (7): 508–517.
- Caldas C., Carneiro F., Lynch H. T., et al. Familial gastric cancer: overview and guidelines for management. J Med Genet, 1999; 36 (12): 873–880.
- Christofori G., Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci, 1999; 24 (2): 73–76.
- Corso G., Marrelli D., Roviello F. E-cadherin germline missense mutations in diffuse gastric cancer. OA Cancer, 2013; 1 (1): 1–4.
- Corso G., Marrelli D., Pascale V., et al. Frequency of CDH1 germline mutations in gastric carcinoma coming from high- and low-risk areas: metanalysis and systematic review of the literature. BMC Cancer, 2012.
- Fitzgerald R., Hardwick R., Huntsman D., et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet, 2010; 47 (7): 436–444.
- Gayther S. A., Gorringe K. L., Ramus S. J., et al. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res, 1998; 15; 58 (18): 4086–4089.
- Graziano F., Humar B., Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann Oncol, 2003; 14 (12): 1705–1713.
- Guilford P. J., Hopkins J. B., Grady W. M., et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Human Mutation, 1999; 14 (3): 249–255.
- Hidetaka Y., Kazuya S., Hiroaki I., et al. Germline alterations in the CDH1 gene in familial gastric cancer in the Japanese population. Cancer Science, 2011; 102 (10): 1782–1788.
- Jacobs G., Hellmig S., Huse K., et al. Polymorphisms in the 3’-untranslated region of the CDH1 gene are a risk factor for primary gastric diffuse large B-cell lymphoma. Haematologica, 2011; 96 (7): 987–995.
- Kaurah P., MacMillan A., Boyd N., et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA, 2007; 297: 2360–2372.
- Keller G., Hartmann A., Mueller J., et al. Denaturing high pressure liquid chromatography (DHPLC) for the analysis of somatic p53 mutations. Lab Invest., 2001; 81 (12): 1735–1737.
- Kluijt I., Sijmons R. H., Hoogerbrugge N., Plukker J. T., et al. Familial gastric cancer: guidelines for diagnosis, treatment and periodic surveillance. Fam Cancer, 2012; 11 (3): 363–369.
- Li Y., Tang Y., Zhou R., et al. Genetic polymorphism in the 3’-untranslated region of the E-cadherin gene is associated with risk of different cancers. Mil. Carcinogenesis, 2011; 50: 857–862.
- Mullins F. M., Dietz L., Lay M., et al. Identification of an intronic single nucleotide polymorphism leading to allele dropout during validation of a CDH1 sequencing assay: implications for designing polymerase chain reaction-based assays. Genet Med., 2007; 9 (11): 752–760.
- Nives P-S. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell International, 2003; 3: 1–7.
- Oliveira C., Sousa S., Pinheiro H., et al. Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression. Gastroenterology, 2009; 136: 2137–2148.
- Pedrazzani C., Corso G., Marelli D., et al. E-cadherin and hereditary diffuse gastric cancer. Surgery, 2007; 142 (5): 645–657.
- Pharoah P., Guilford P., Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology, 2001; 121: 1348–1353.
- Takashima H., Boerkoel C. F., Lupski J. R., Screening for mutations in a genetically heterogeneous disorder: DHPLC versus DNA sequence for mutation detection in multiple genes causing Charcot-Marie-Tooth neuropathy. Genetics In Medicine, 2001; 3 (5): 335–342.
- Wagner A. O., Malin C., Illmer P. Application of denaturing high-performance liquid chromatography in microbial ecology: fermentor sludge, compost, and soil community profiling. Applied and Environmental Microbiology, 2009; 75 (4): 956–964.
- Zhang X. F., Wang Y. M., Cao Y. Y. Association of CDH1 single nucleotide polymorphisms with susceptibility to esophageal squamous cell carcinomas and gastric cardia carcinomas. Diseases of the Esophagus, 2008; 21: 21–29.



