Occurrence of Legionella Pneumophila in Water Distribution Systems in Dental Practices in Latvia
Abstract
Legionella pneumophila is the major agent of Legionnaire’s disease and Pontiac fever. Legionellosis are normally acquired by inhalation or aspiration of legionellae from a contaminated environmental source. The pathogens present in dental unit waterline could be spread by aerosols created by dental equipment, presenting a risk for both a patient and members of a dental team.
The aim of the study was to investigate the occurrence of Legionella contamination of water distribution systems in dental practices. A total of 185 samples were collected from 74 dental practices. Samples were taken from water taps in dental practices (n = 79) and from dental unit waterline (n = 106). Overall, 20 out of 74 (27 %) of dental practices were found Legionella pneumophila positive. Occurrence of Legionella pneumophila was significantly higher in samples from water taps than in samples from dental unit waterlines – 25 of 79 (25 %) and 5 of 106 (5 %), accordingly. From all Legionella pneumophila positive samples, 23 (92 %) represented L. pneumophila serogroup 2–15. Two samples from dental practices in Rīga were contaminated with L. pneumophila serogroup 1. The level of contamination of samples from water taps ranged from 2 × 10² CFU/L to 1.1 × 104 CFU/L, and the level of contamination of samples from dental unit waterlines ranged from 3 × 10² CFU/L to 2.4 × 10³ CFU/L. Both samples from water taps and dental unit waterlines were positive in three dental practices (4 %). In two cases, samples from water taps were negative, though Legionella pneumophila was found in samples from dental unit waterlines.
The study showed no correlation between the year of installation of dental unit and occurrence of Legionella pneumophila, since it was isolated from samples taken from dental units installed in the year 1998 and up to the year 2013. Legionella pneumophila was found in one dental practice dental unit waterline with independent distilled water supplying system.
Introduction
Legionella pneumophila is a facultative intracellular bacterium that multiplies within phagocytic cells [Diederen, 2008]. Legionella pneumophila is the major agent of Legionnaire’s disease and Pontiac fever. Legionellosis are normally acquired by inhalation or aspiration of legionellae from a contaminated environmental source. Moreover, Legionella strains can survive in moist environments for long periods and can be ubiquitously found in natural moist environments and man-made systems. In natural environments, Legionella is present in low density but its concentration can significantly increase in artificial habitats depending on the type of materials, on the presence of biofilms and available nutrients [Veronesi, 2007]. Bacterial biofilm in dental unit waterlines (DUWL) is a widespread problem [Tuttlebee, 2002]. Each dental chair unit (DCU) is equipped with an elaborate loom of interconnected narrow-bore flexible plastic tubing called dental unit waterlines (DUWLs), which supply water to all of the DCU-supplied instruments [O’Donnel, 2011]. The water used in DUWL acts as a coolant for high speed drills and as irrigant during dental procedures, most often it is supplied directly from municipal water supplies [Walker, 2004]. The general problem of microbial contamination of DUWL is well known [Atlas, 1995; Pankhurst, 1998]. Due to the texture and composition of the plastic tubing, microbial biofilms form readily, resulting as high bacterial contaminations in outputs water. The pathogens present in DUWL could be spread by aerosols created by dental hand-pieces, presenting a risk for both a patient and members of a dental team [Laheij, 2012].
Aim
The aim of the study was to investigate the occurrence of Legionella contamination of water distribution systems in dental practices, and whether dental treatment might pose a risk for patients and for dental team. In addition, analysis of hot tap water samples for presence of Legionella were carried out in order to assess the prevalence of Legionella in water supply system in the entire building.
Material and methods
A total of 185 samples were collected from 74 dental practices from February 2014 until June 2014. Samples were taken in Rīga (n = 71) and four regions of Latvia, randomly representing Latgale (n = 40), Kurzeme (n = 34), Vidzeme (n = 25) and Zemgale (n = 15). The samples were taken from water taps in dental practices (n = 79) and from dental unit waterline (n = 106). Water samples were collected in sterile bottles before routine working hours. At least two samples were collected in each dental practice, one sample from DUWL (cup filler) and one hot tap water sample from the sink in the same room. In dental practices, which have more than one or two dental chair units, up to 10 DUWL samples were taken per practice. During the sampling, the dental personnel was asked for additional information about the year of installation of DCU and methods for treatment of DUWL incoming water.
Isolation and identification of Legionella pneumophila was carried out by using standard ISO 11731. One litre of water sample was filtrated and concentrated using membrane filtration with 0.45 μm pore-size polyamide filter (Millipore, USA). The filter membranes were cut into pieces and resuspended in 5 ml sterile distilled water, then shaken for two minutes (Vortex Genie) and kept in room temperature for 10 minutes. A total of three 0.1 ml untreated, heat-treated and acid-treated aliquots of the sample were spread on Buffered Charcoal Yeast extract medium (GVPC, Oxoid, UK). The plates were incubated at 36 °C in a humidified environment for 10 days, and examined every day beginning on day 3. At least three characteristic colonies from each GVPC plate were selected for subculture onto plates Buffered Charcoal Extract agar medium with L-cysteine (BCYE, OXOID, UK) and Buffered Charcoal Extract agar medium without L-cysteine (BCYE-Cys, OXOID, UK) and incubated for at least 48 hours at 36 °C. Colonies grown on BCYE were subsequently identified by latex agglutination test (Microscreen Legionella CE, Microgen Biologics, UK). Legionella Rapid Latex Test Kit allows for separate identification of L. pneumophila serogroup 1 and serogroups 2–15 and identification of 10 non-Legionella pneumophila species. Colonies from all plates were counted, confirmed and the estimated number of Legionella was expressed as CFU/litre Legionella species and serogroup.
Microbiological analysis was carried out in Laboratory of Medical Microbiology (Institute of Food Safety, Animal Health and Environment “BIOR”).
Results
Overall, 20 out of 74 (27 %) dental practices were found Legionella positive (Fig. 1, Fig. 2). However, Legionella was not found in samples from dental practices in Zemgale. In other districts, the occurrence of Legionella ranged from 13% in Latgale up to 48 % in Rīga (Table 1).
Samples were taken in different administrative districts of Rīga (Figure 2), where Legionella was found in 9 of 15 (60 %) administrative districts.
Overall, Legionella was isolated in 25 out of 185 samples (14 %). The occurrence of Legionella was significantly higher (p = 0.04) in samples from water taps than in samples from dental unit waterlines – 25 of 79 (25 %) and 5 of 106 (5 %), accordingly (Table 1).
From all Legionella positive samples, 23 (92 %) represented L. pneumophila serogroup 2–15. Two samples from dental practices in Rīga were contaminated with L. pneumophila serogroup 1. The level of contamination of samples from water taps ranged from 2 × 10² CFU/L to 1.1 × 104 CFU/L, and the level of contamination of samples from dental unit waterlines ranged from 3 × 10² CFU/L to 2.4 × 10³ CFU/L (Table 2).
Both samples from water taps and dental unit waterlines were positive in three dental practices (4 %). In two cases, samples from water taps were negative, though Legionella was found in samples from dental unit waterlines.
Table 1. Occurrence of Legionella in samples from water taps and dental equipment in regions of Latvia
| District | Number of dental practices, n | Number of practices with at least one positive sample, n (%) | Samples from water taps. Number of samples / positive samples, n (%) | Samples from dental unit waterline. Number of samples / positive samples, n (%) |
|---|---|---|---|---|
| Rīga | 23 | 11 (48) | 27 / 11 (41 %) | 44 / 3 (7 %) |
| Latgale | 16 | 2 (13) | 16 / 2 (13 %) | 24 / 0 (0 %) |
| Kurzeme | 15 | 3 (20) | 15 / 3 (20 %) | 19 / 2 (11 %) |
| Vidzeme | 13 | 4 (31) | 14 / 4 (31 %) | 11* / 0 (0 %) |
| Zemgale | 7 | 0 (0) | 7 / 0 (0 %) | 8 / 0 (0 %) |
| TOTAL | 74 | 20 (27) | 79 / 20 (25 %) | 106 / 5 (5 %) |
* In two dental practices in Vidzeme, only tap water samples were taken.
Figure 1. Sampling points and Legionella positive results in Latvia
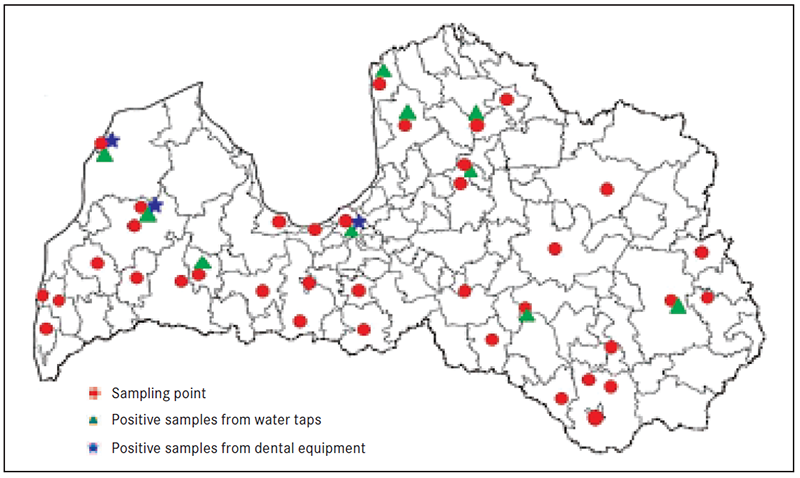
Figure 2. Sampling points and Legionella positive results in districts Rīga
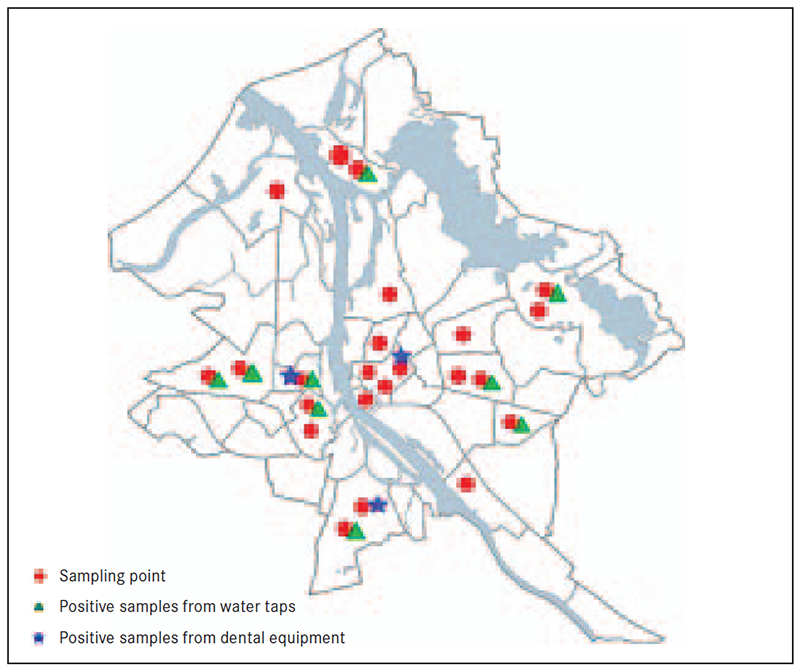
Table 2. Legionella positive samples and level of colonisation
| ID number | District | Samples from hot water taps | Samples from dental unit waterline | ||
|---|---|---|---|---|---|
| CFU/L | Serogroup | CFU/L | Serogroup | ||
| 19 | Kurzeme | 4 × 10³ | 2–15 | 1 × 10³ | 2–15 |
| 20 | Vidzeme | 6 × 10³ | 2–15 | ND | ND |
| 21 | Latgale | 9 × 10² | 2–15 | ND | ND |
| 22 | Vidzeme | 3.5 × 10³ | 2–15 | ND | ND |
| 36 | Vidzeme | 1 × 10³ | 2–15 | ND | ND |
| 37 | Vidzeme | 6 × 10² | 2–15 | ND | ND |
| 38 | Latgale | 3 × 10³ | 2–15 | ND | ND |
| 39 | Kurzeme | 8 × 10² | 2–15 | 3 × 10² | 2–15 |
| 46 | Rīga | 7 × 10³ | 2–15 | ND | ND |
| 47 | Rīga | 1.1 × 104 | 2–15 | ND | ND |
| 49 | Rīga | 8 × 10³ | 2–15 | ND | ND |
| 50 | Rīga | ND | ND | 2.4 × 10³ | 2–15 |
| 51 | Rīga | 2.5 × 10³ | 1 | ND | ND |
| 54 | Rīga | 4 × 10² | 2–15 | ND | ND |
| 58 | Rīga | 8 × 10² | 2–15 | ND | ND |
| 61 | Rīga | 2 × 10² | 2–15 | ND | ND |
| 4 × 10² | 2–15 | ND | ND | ||
| 9 × 10³ | 2–15 | 1.1 × 10³ | 2–15 | ||
| 66 | Rīga | 7 × 10² | 2–15 | ND | ND |
| 68 | Rīga | 6 × 10³ | 2–15 | ND | ND |
| 70 | Rīga | ND | ND | 1.2 × 10³ | 2–15 |
| 74 | Kurzeme | 2 × 10³ | 2–15 | ND | ND |
* ND – not detected.
Table 2. Installation period of dental chair units included in sampling plan
| District | Period of installation, years | Average age of DCU, years |
|---|---|---|
| Rīga | 2000–2014 | 4.7 |
| Latgale | 2000–2009 | 8.3 |
| Kurzeme | 1998–2014 | 6.4 |
| Vidzeme | 1995–2014 | 7.5 |
| Zemgale | 1997–2013 | 6.9 |
The study showed no correlation between the year of installation of dental unit and the occurrence of Legionella, since it was isolated from samples taken from dental units installed in years between 1998 and 2013. Some DCUs use independent water reservoir bottles to provide distilled water to the DUWLs.
Thus, Legionella was found in one dental practice DUWL with such water supplying system. The only method used for municipal water additional treatment, were filters. No influence of additional filters on occurrence of Legionella was observed.
Discussion
This study showed that 27 % of dental practices had at least one Legionella positive sample. Overall, Legionella was found in 5 of 106 DUWL samples, which is significantly lower than in other countries with a higher average annual temperature, where the occurrence of Legionella in DUWL systems varied from 16.1 % in Greece [Mavridou, 2006], 33 % in South Africa [Singh, 2005], 33.3 % in Italy [Montagna, 2006] and 86.7 % in Jordan [Ma’ayeh, 2008].
Water temperature could be the main reason for significant differences. It is difficult to maintain cool water temperature below 20 °C in countries with high average air temperature. Optimum temperature range for proliferation of legionellae is 32–35 °C [Levesque, 2004; Wadovsky, 1985]; however, in Latvia, cold water temperature rarely exceeds 20 °C. In some countries with similar climate, the results may vary. No Legionella positive dental unit reservoir samples were found in Poland [Szymanska, 2004]; in London and Northern Ireland the prevalence of Legionella was very low (0.37%) [Pankhurst, 2003]; however, a significantly higher occurrence was observed in Sweden (15 %) [Dahlen, 2009], Switzerland (20 %) [Barben, 2009] and Germany (27.8 %) [Arvand, 2013]. Differences in the occurrence of Legionella can be explained by different sampling strategies. In the retrospective study, DUWL samples were mainly taken from cup-fillers, while in other researches samples were taken from high-speed hand-piece tube, syringe or micromotors. It has been confirmed that cup-filler samples can be twice less contaminated with Legionella than samples from instrument channels [Arvand, 2013].
Some DCUs use independent water reservoir bottles to provide water to the DUWLs. These bottles were manually filled with distilled or sterile water.
One of Legionella positive samples was taken in dental practice, which does not use municipal water, but DCU is supplied by distilled water from a single reservoir. However, it does not protect against contamination. Even DUWL supplied by sterile or distilled water, at the moment of filling will become colonised to the same extent as those supplied by tap water. Once the bacteria gained access to the system, there will be enough nutrients from the plastic tubing and the turnover of the bacteria themselves to support biofilm growth. This does create difficulties for some practitioners, despite the use of sterile water source [Walker, 2004].
Our results showed no correlation between the year of installation of dental unit and the occurrence of Legionella; it was isolated from samples taken from dental units installed between years 1998 and 2013. Most DCUs often are not used for more than 12 hours per day, 5 days per week, and thus water stagnation is a significant contributory factor to DUWLs output water contamination [O’Donnell, 2011]. Historically, the majority of DUWL have been supplied by municipal tap water, which is still the case today in Latvia. With such systems, even within 5 days of installation, the microbial counts can reach 2.0 × 105 CFU/ml in the water at the distal outlets [Walker, 2004; Barbeau, 1996]. Complex design of dental chair equipment, resulting in the stagnation of water within the equipment lines where bacteria, including Legionella pneumophila could proliferate within biofilm is a major factor affecting microbial contamination of water lines [Smith, 2002]. DCU manufacturers can significantly contribute to controlling the problem of DUWL biofilm [Coleman, 2007].
The occurrence of L. pneumophila was considerably higher in hot tap water (25 %) compared to other European countries, where the occurrence of Legionella in water distribution systems varied from 22.6 % in Italy [Borella, 2004], 26 % in Germany [Zietz, 2001] to 30 % in Finland [Zacheus, 1994].
A total of 15 dental practices, where Legionella was found in hot tap water samples, were not contaminated in DUWL. This may suggest that incoming municipal water could be a source of infection for DUWL biofilms, which is in accordance with previous studies [Valcina, 2013] and using other sampling strategies and methods of analysis, Legionella prevalence in DUWL could be higher. However, it has to be emphasised that the classical cultivation method used in this study did not allow determining the presence of non-cultivable legionellae [Delgado-Viscogliosi, 2005].
Statistically significant differences (p = 0.02) were observed in the distribution of L. pneumophila in different districts of Latvia. Zemgale was the only region where Legionella was not detected in any sample.
From all L. pneumophila positive samples, 8 % represented L. pneumophila serogroup 1 and 92 % L. pneumophila serogroup 2–15. Both cases of serogroup 1 were observed in Rīga, in territories, which received treated surface water. The data are consistent with results of other studies. In Poland, L. pneumophila 2–15 serogroup was isolated from 73 % and serogroup 1 from 19.8 % of Legionella spp. positive samples [Stojek, 2011], in Italy 75.6 % and 22.6 %, respectively [Borella, 2004].
Currently, only one case has been reported about an 82-year-old woman who died of Legionnaires disease in Italy in 2011 [Ricci, 2011]. Nevertheless, dental personnel and the increasing number of immunocompromised dental patients that present routinely at dental surgeries are being exposed to potentially opportunistic pathogenic bacteria through ingestion and inhalation of dental unit water [Walker, 2004]. The potential occupational hazard to a dental team is considered greater than that of the patient population due to sustained and daily contact with contaminated DUWL aerosols [Pankhurst, 2007].
Conclusions
- Our study showed that several dental unit water lines contained Legionella pneumophila (5 %), which poses a risk for both patients and dental team. However, the actual risk of legionellosis based on our results has to be studied further.
- High contamination of hot tap water with Legionella pneumophila (25 %) can indicate that incoming water may cause a threat to dental unit water line systems.
- Regular monitoring of microbial contamination of dental unit waterlines is essential to control and reduce the microbial burden within dental unit water lines as well as to highlight the risk of occupational exposure in general dental practices.
Acknowledgements
Laboratory of Medical Microbiology, Customer Service experts and “BIOR” Regional laboratories (Institute of Food Safety, Animal Health and Environment “BIOR”) are acknowledged for their tehnical support.
References
- Arwand M., Hack A. Microbial contamination of dental unit waterlines in dental practices in Hesse, Germany: A cross sectional study // European Journal of Microbiology and Immunology, 2013; 3 (1): 49–52.
- Atlas R. M., Williams J. F., Huntington M. K. Legionella contamination of dental-unit waters // Applied and Environmental Microbiology, 1995; 61 (4): 1208–1213.
- Barbeau J., Tanguay R., Faucher E,. et al. Multiparametric analysis of waterline contamination in dental units // Applied and Environmental Microbiology, 1996; 62: 3954–3959.
- Barben J., Kuehni E., Schimd J. Water quality in dental chair units // Schweiz Monatsschr Zahnmed, 2009; 119 (10): 976–980.
- Borella P., Montagna M. T., Romano-Spica V., et al. Legionella infection risk from domestic hot water // Emerging Infectious Diseases, 2004; 10 (3): 457–464.
- Coleman D. C., O’Donnell M. J., Shore A. C., et al. The role of manufacturers in reducing biofilms in dental chair waterlines // Journal of Dentistry, 2007; 35: 701–711.
- Dahlen G., Alenas-Jarl E., Hjort G. Water quality in water lines of dental units in the public dental health service in Göteborg, Sweden // Swedish Dental Journal, 2009; 33 (4): 161–172.
- Diederen B. Legionela spp. and Legionnaires’ disease // Journal of Infection, 2008; 56: 1–12.
- Delgado-Viscoliosi P., Simonart T., Parent V., et al. Rapid method for enumeration of viable Legionella pneumophila and other Legionella spp. in water // Applied and Environmental Microbiology, 2005; 71: 4086–4096.
- Laheij A. M. G. A., Kistler J. O., Belibaskis G. N., et al. Healthcare-associated viral and bacterial infections in dentistry // Journal of Oral Microbiology, 2012; 4: 17659, 1–10.
- Levesque B., Lavoie M., Joly J. Residential water heater temperature 49 or 60 degrees Celsius? // Canadian Journal of Infectious Disease, 2004; 15 (1): 11–12.
- Ma’ayeh S. Y., Al-Hiyasat A. S., Hindiyeh M. Y., Khader Y. S. Legionella pneumophila contamination of a dental unit water line system in a dental teaching centre // International Journal of Dental Hygiene, 2008; 6 (1): 48–55.
- Mavridou A., Kamma J., Mandilara G., et al. Microbial risk assessment of dental unit water systems in general dental practice in Greece // Water Science and Technology, 2006; 54 (3): 269–273.
- Montagna M. T., Tato D., Napoli C., et al. Pilot study on the presence of Legionella spp. in 6 Italian cities’ dental units // Annali di Igiene, 2006; 18 (4): 297–303.
- O`Donnell M. J., Boyle M. A., Russell R. J., Coleman D. C. Management of dental unit waterline biofilms in the 21st century // Future Medicine, 2011; 6 (10): 1209–1226.
- Pankhurst C. L., Coulter W., Philpott-Howard J. J., et al. Prevalence of legionella waterline contamination and Legionella pneumophila antibodies in general dental practitioners in London and rural Northern Ireland // British Dental Journal, 2003; 195 (10): 591–594.
- Pankhurst C. L., Coulter W. A. Do contaminated dental unit waterlines pose a risk of infection? // Journal of Dentistry, 2007; 35: 712–720.
- Pankhurst C. L., Johnson N. W., Woods R. G. Microbial contamination of dental unit waterlines: The scientific argument // International Dental Journal, 1998; 48: 359–368.
- Ricci M. L., Fontana S., Pinci F., et al. Pneumonia associated with a dental unit waterline // The Lancet, 2012; 379: 684.
- Singh T., Coogan M. M. Isolation of pathogenic Legionella species and legionella-laden amoebae in dental unit waterlines // Journal of Hospital Infection, 2005; 61: 257–262.
- Smith A. J., McHugh S., McCormick L., et al. A cross sectional study of water quality from dental unit water lines in dental practices in the West Scotland // British Dental Journal, 2002; 193 (11): 645–648.
- Stojek N. M., Dutkiewicz J. Co-existence of Legionella and other gram-negative bacteria in potable water from various rural and urban sources // Annals of Agricultural and Environmental Medicine, 2011; 18 (2): 330–334.
- Szymanska J., Wdowiak L., Puacz E., Stojek N. M. Microbial quality of water in dental unit reservoirs // Annals of Agricultural and Environmental Medicine, 2004; 11 (2): 355–358.
- Tuttlebee C. M., O’Donnell M. J., Keane C. T., et al. Effective control of dental chair unit waterline biofilm and marked reduction of bacterial contamination of output water using two peroxide-based disinfectants // Journal of Hospital Infection, 2002; 52: 192–205.
- Valcina O., Pule D., Makarova S., et al. Occurrence of Legionella pneumophila in hot potable water in Latvia // Journal of Environmental Science and Engineering, 2013; 2 (3): 135–140.
- Veronesi L., Capobianco E., Affanni P., et al. Legionella contamination in the water system of hospital dental settings // Acta Biomed, 2007; 78: 117–122.
- Wadovsky R. M., Wolford R., McNamara A. M., Yee R. B. Effect of temperature, pH and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water // Applied and Environmental Microbiology, 1985; 49 (5): 1197–1205.
- Walker J. T., Marsh P. D. A review of biofilms and their role in microbial contamination of dental unit water systems (DUWS) // International Biodeterioration and Biodegradation, 2004; 54: 87–98.
- Zacheus O. M., Martikainen P. J. Occurrence of legionellae in hot water distribution sustems of Finland apartment buildings // Canadian Journal of Microbiology, 1994; 40: 993–999.
- Zietz B., Wiese J., Brengelmann F., Dunkelberg H. Presence of Legionellaceae in warm water supplies and typing of strains by polymerase chain reaction // Epidemiol Infect, 2001; 126: 147–152.



