Innate and Acquired Immunity Features of Psoriatic Nails: Expression of hβD-2, hβD-3, hβD-4 and IL-1, IL-6, IL-10
Abstract
Psoriasis is a chronic inflammatory skin disease where nail involvement is an important aspect although often underestimated. Our knowledge of nail immunology is still very scarce. To develop more targeted therapies, it is relevant to understand the immunologic mechanisms of psoriatic nails.
The aim of the study was to determine the presence and distribution of human beta defensins and cytokines in psoriatic nail apparatus biopsies comparing with healthy nails.
We obtained 8 biopsies of nail psoriasis and control group with material of 5 healthy nail apparatus from fresh cadavers with a punch method and fixed in Stefanini’s solution, dehydrated and embedded in paraffin. Subsequently we performed staining with haematoxylin and eosin and immunohistochemistry for human β-defensins (hβD-2, hβD-3 and hβD-4) as well as interleukins (IL-1α, IL-6 and IL-10) and evaluated the results semi-quantitatively grading the intensity of positively stained structures in the visual field.
Both in psoriatic nails and the control group, hβD-2 and hβD-3, not hβD-4, was positively expressed with predominance in psoriatic nails. In psoriatic nails, IL-1α was absent, while it was moderately positive in control group. Structures positive for IL-6 and IL-10 in psoriatic nails were found in greater extent compared to the control group.
The increased expression of hβD-2 and hβD-3 in the psoriatic nail highlights the potential role of antimicrobial peptides for the innate immunity involvement in the pathogenesis of nail psoriasis.
In psoriatic nails, the pro-inflammatory (IL-1α) cytokine expression is decreased, but expression of IL-6 as well as the anti-inflammatory cytokine IL-10 is increased indicating stimulation of nail growth and increase of anti-inflammatory immunity.
Introduction
Around 3 % of the population has psoriasis, and the nails are involved in up to half of the patients with psoriasis, besides often the skin surrounding the affected nails remains “normal”. Although nails are involved in tactile sensitivity, ensure small object retrieving, plays aesthetic role, its impact on patients’ quality of life has often been overlooked [21].
Despite its high prevalence and significant impact on the patients’ quality of life, nail psoriasis treatment remains a challenge for physicians and no consistent approach has been advocated [29].
As conventional therapies are often ineffective or inconvenient for patients, the effects of biologic agents on nail psoriasis have been investigated. Thus, the effectiveness of biological treatment in nail psoriasis appears to offer great promise for the future management of this distressing condition.
Even the understanding of healthy human nail immunology is scarce; regarding nail psoriasis there is only fragmentary knowledge that has been concluded from the results of the efficacy of the biologic treatment. The mechanisms of nail psoriasis should be investigated to apply targeted treatment options. It should not be ignored that although the nail is an appendage of the skin, there are important distinctive immunological features between the nail and skin, therefore the characteristics of the skin may not be automatically referred to the nail as well. Clinical manifestations of nail psoriasis vary according to the part of the nail affected by the inflammation.
Histologically, the changes are similar to those observed in psoriasis plaques on the skin. However, in contrast to other areas of the skin, the nail commonly shows evidence of spongiosis and serous exudates, and hypergranulosis may be observed [25].
The normal nail immune system of human infants shows some similarities to human hair immune system, both exhibiting defined compartments that represent as the sites of relative immune privilege [9, 22, 6]. It has been proclaimed that in the human nail apparatus there are decreased levels of key protagonists (natural killers and mast cells) of innate immunity [9]. Therefore an important role that still has to be clarified in the human nail innate immunity may be played by the antimicrobial peptides (AMPs) of which cathelicidins and β-defensins are the most well-characterised AMPs found in the human skin [2]. However, in the human nail apparatus from antimicrobial peptides only the presence of cathelicidin group peptide LL-37 has been described [16].
Aim
The aim of the study was to determine the presence and distribution of human beta defensins (hβDs) – hβD-2, hβD-3 and hβD-4 and interleukins (IL) – IL-1α, IL-6, IL-10 – in psoriatic nail apparatus biopsies compared with healthy nails.
Material and methods
The pilot study included eight (n = 8) patients’ aged 18 to 70 years nail unit tissue samples obtained with a punch (diameter of five millimetres) biopsy technique from the nail unit according to our selection criteria with pathohistologically confirmed diagnosis of nail psoriasis. Exclusion criteria were other skin or its derivate diseases than psoriasis (including coexisting onychomycosis samples with negative culture for fungi and negative Periodic Acid Schiff (PAS) reaction were selected) in anamnesis, local or systemic anti-bacterial, anti-fungal, anti-inflammatory, immunosuppressive or exposure to an artificial UV radiation source within the last month.
As a control material five (n = 5) samples of fresh (samples were obtained within twelve hours after death) cadaverous’ nail units were obtained. The included sample material was clinically unaffected nails’ units with pathohistological appearance of normal nail apparatus and negative Periodic Acid Schiff reaction; without skin diseases clinically or in anamnesis, with no anti-bacterial or immunosuppressive treatment within the last two weeks.
The study was approved by the Ethical Committee at Rīga Stradiņš University (permit issued on 26.01.2012.)
Human nail biopsies were fixed in Stefanini’s solution, dehydrated, and embedded in paraffin. Four-micrometre-thick sections were prepared from each tissue specimen and stained routinely with haematoxylin and eosin; Periodic Acid Schiff reaction was performed.
Immunohistochemistry (IMH) method was performed for human beta defensin-2 (hβD-2) (VJU01.1 : 100; R & D Systems, USA), human beta defensin-3 (hβD-3), human beta defensin-4 (hβD-4), human 1 alpha interleukin (Il-1α) (SC-9983, 1 : 50; Santa Cruz Biotechnology, USA), interleukin 6 (IL-6) (NYRhIL6, 1 : 50; Santa Cruz Biotechnology, USA) and interleukin 10 (IL-10) (AB34843, 1 : 400, Abcam, UK).
The results were evaluated semiquantitatively grading the appearance of positively stained structures in the visual field [27]. Few positive structures in the visual field were labelled with +, moderate number of positive structures in the visual field was labelled with ++, numerous positive structures in the visual field were labelled with +++ and abundance of positive structures in the visual field was marked with ++++.
For statistical analysis, non-parametric statistics with Mann–Whitney U-test were used.
For visual illustration of our findings, we used Leica DC 300F digital camera and image processing, and analysis software Image Pro Plus (Media Cybernetics, Inc., Rockville, MD, USA).
Results
Hyperkeratosis, parakeratosis, spongiosis, focal hypergranulosis and dilated vessels in the papillary dermis, as well as infiltration of neutrophils were observed in the psoriatic nail bed and connective tissue.
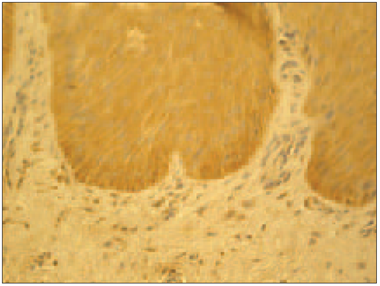
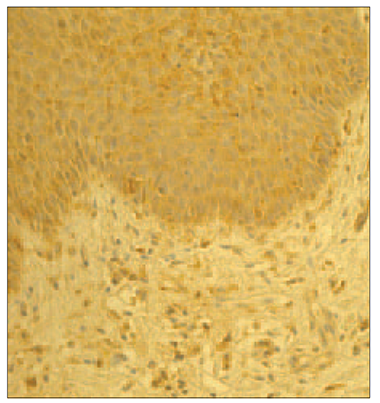
In psoriatic nails hβD-2 and hβD-3 positive structures observed in epithelia varied from numerous (+++) to abundance (++++) in view field (Figure 1, Table 1); in connective tissue hβD-2 was moderate (++), but for hβD-3 there were few positive cells (+). In the nail bed of healthy nails, there was moderate (++) amount of hβD-2 and hβD-3 containing structures (Figure 2). Structures containing hβD-2 and hβD-3 were absent in the connective tissue of the healthy nail. In control group, hβD-2 and hβD-3 positive epithelial cells of the nail bed displayed a patchy distribution prevailing close to the nail plate whereas in psoriatic nails it was evenly expressed both in the nail bed and in connective tissue. Noticeably more positive structures stained for hβD-2 and hβD-3 could be observed per visual field in psoriatic nails. Structures positive for hβD-4 were absent in psoriatic nails as well as in control group nails.
Statistically significant difference (p < 0.05) comparing appearance of hβD-2, hβD-3 and hβD-4 in psoriatic nail group with the control group nails could be observed for all the defensins both in nail bed and in connective tissue (Table 1).
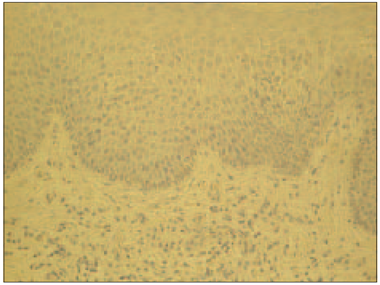
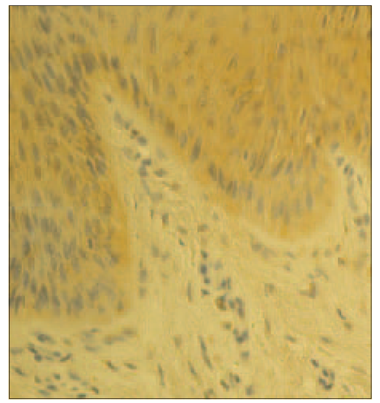
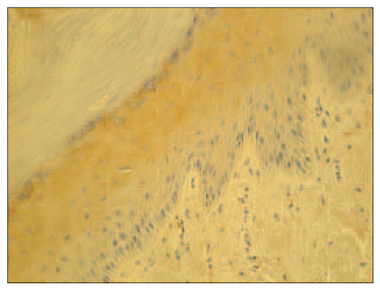
In psoriatic nails, IL-1α positive structures were absent (Figure 3), while in the control group there was moderate (++) number of IL-1α positive cells in the nail bed and few (+) IL-1α positive cells in the connective tissue (Figure 4).
Table 1. Statistic analysis of cytokines and anti-microbial peptides appearance data
| Method | Psoriasis affected nails | Control group nails | ||||||
|---|---|---|---|---|---|---|---|---|
| Nail bed | Mann– Whitney U test | p | Connective tissue | Mann– Whitney U test | p | Nail bed | Connective tissue | |
| IL-1 | 0 | 15.0 | 0.435 | 0 | 0 | 0.010 | ++ | + |
| IL-6 | +++ | 6.0 | 0.021 | ++ | 2.0 | 0.014 | ++ | 0 |
| IL-10 | +++ | 5.0 | 0.018 | + | 8.0 | 0.143 | ++ | + |
| hβD-2 | +++ | 2.5 | 0.006 | ++ | 0.0 | 0.002 | ++ | 0 |
| hβD-3 | +++ | 5.0 | 0.024 | + | 5.0 | 0.033 | ++ | 0 |
| hβD-4 | 0 | 0.0 | 0.000 | 0 | 0.0 | 0.000 | 0 | 0 |
Designation of semiquantative method: 0 – negative reaction; 0 / + – few positive elements in some preparations; + – few positive structures in the visual field; ++ – moderate number of positive structures in visual field; +++ – numerous positive structures in the visual field; ++++ – abundance of positive structures in the visual field.
Mann–Whitney U-test: displays the ranking differences between the control group and studied group, that is, the lower the Mann–Whitney U test, the higher the ranking difference.
P-significance: if significance is < 5 %, then the probability to accept zero hypothesis is applied – the values in the groups are the same size, is less than 5 %, i.e., saying, that the hypothesis of the uniformity of value size in groups may be indicated at 5 % confidence level.
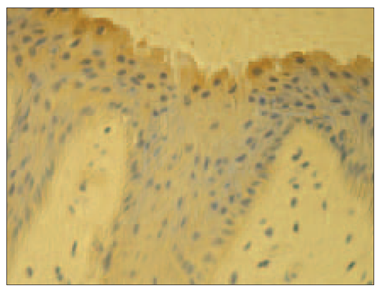
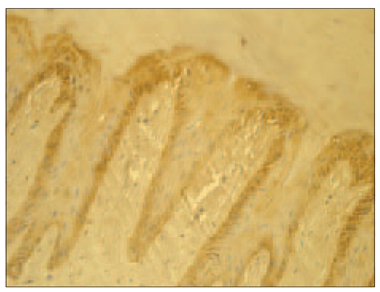
There were more IL-6 positive structures in psoriatic nails than in the control group nails (Table 1). Numerous (+++) IL-6 positive epithelial cells were observed in the psoriatic nails’ bed and moderate (++) in connective tissue cells (Figure 5). Meanwhile in the control group there were only few (+) IL-6 positive cells (Figure 6).
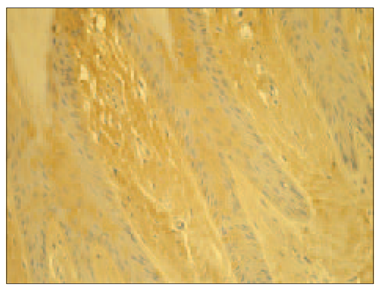
There were more IL-10 positive structures in psoriatic nails than in the control group nails (Table 1). In psoriatic nails, IL-10 appeared positive in moderate (++) to numerous (+++) numbers in the nail bed with predominance in the basal layer, but only few (+) IL-10 positive cells were found in the connective tissue (Figure 7), whereas in the control group it showed moderate (++) number of IL-10 positive epithelial cells in the nail bed and varied from few (+) to absence (0) in the connective tissue (Figure 8). Statistically significant difference (p < 0.05) comparing interleukin appearance in psoriatic nail group with the control could be observed for IL-6 both in nail bed (p = 0.021) and connective tissue (p = 0.014), IL-1 in connective tissue (p = 0.010) and IL-10 in nail bed (p = 0.018) (Table 1).
| Figure 1. Abundance of hβD-2 containing cells in the psoriatic nail (hβD-2 IMH, × 250) | Figure 3. Absence of IL-1 expression in the psoriatic nail (IL-1 IMH, × 250) |
|---|---|
|
|
| Figure 2. Few hβD-2 containing cells in the control group nail apparatus (hβD-2 IMH, × 250) | Figure 4. Moderate numbers of IL-1 containing cells in the control group nail (IL-1 IMH, × 250) |
|
|
| Figure 5. Abundance of IL-6positive structures in epithelia and moderate amount in connective tissue in psoriatic nail (IL-6 IMH, × 250) | Figure 7. Abundance of IL-10 positive cells in the nail bed and few positive structures in connective tissue in the psoriatic nail (IL-10 IMH, × 250) |
|
|
| Figure 6. Moderate IL-6 positive structures in epithelia and negative expression in connective tissue in control group nail (IL-6 IMH, × 250) | Figure 8. Moderate amount of IL-10 positive cells in epithelia in the control group nail (IL-10 IMH, × 250) |
|
|
Discussion
It has been described that human nail matrix represents a site of relative immune privilege, partly due to the low number of the potent immunosuppressants (ACTH, a-MSH, IGF-1, TGF-b1). The relative immune privilege of the proximal nail matrix may serve to suppress inflammatory/autoimmune damage of the most critical component of the actual nail growth to prevent the loss of nails [9]. However, there is no functional evidence that defines compartments of the nail apparatus immune privilege. In addition, the specific arrangement of the nail immune system (NIS) is poorly understood.
It should be considered, whether a well-developed immune system might be promoted by components of innate immune system, in part with the expression of anti-microbial peptides. In nail apparatus, from anti-microbial peptides only cathelicidin LL-37 has been described [16]. Expression of hβD-2 and hβD-3, but not hβD-4, was detected both in healthy and psoriatic nails of our study group patients. Furthermore, in control group hβD-2 and hβD-3 positive structures in the epithelia of the nail bed had a patchy distribution prevailing close to the nail plate. These findings suggest that a significant part of the human nail immune system could be ensured by anti-microbial peptides of the defensin group. Those peptides in healthy nails were distributed mainly closer to the nail plate where the microbial agent is exposed. Thus, we hypothesised that in addition to anti-microbial peptide cathelicidin LL-37 also other antimicrobial peptides that are capable to ensure an effective innate immunity defence system in the presence of intense exposure to various microbiological agents, are also present in nails, despite the relative immune privilege in the nail matrix [9, 16].
In several studies exploring psoriatic skin, it has been shown that AMPs such as hβD-2, psoriasin, and LL-37 are strongly over-expressed in psoriatic plaques [8, 31, 16, 19, 20]. Similarly, in all our patients’ samples of a chronic inflammatory nail disease by psoriasis affected nails noticeably more positive structures for hβD-2 and hβD-3 were expressed evenly both in the nail bed and connective tissue. However, it is increasingly evident that these peptides not only act as endogenous antibiotics but also display additional roles, such as regulation of inflammatory and immune responses, chemo-attracting immune or inflammatory cells to wound or infection/inflammation sites, acceleration of angiogenesis, promotion of wound healing, and re-epithelisation, and many others that still have to be explored [13].
In addition, it has been suggested that in the psoriatic skin an endogenous antimicrobial peptide may play an important role in breaking innate tolerance that could induce auto-immunity in psoriasis [4]. On the other hand, the pathogenesis of nail psoriasis has been proposed to have an auto-inflammatory basis rather than auto-immune where subclinical micro-damage of the enthesis results in a diffuse soft tissue inflammation including nail lesions. The consequences of inflammatory disease in this anatomical region should be considered in understanding the development of nail psoriasis [11]. Therefore, to explore the cytokine expression in psoriatic nails compared with healthy nails, we chose cytokines the role of which has well been described in psoriatic skin plaque – IL-1, IL-6 and IL-10.
Although IL-1 expression in the psoriatic epidermis appears altered, data on this finding are often conflicting. Some studies showed that IL-1α levels in psoriatic lesions were decreased or below detection limits in comparison to non-lesional and healthy skin [15, 3, 32] whereas increased levels of IL-1α were noted in supernatants of monocyte cultures obtained from patients with psoriasis [15]. We observed that IL-1α was expressed to a higher amount in the epithelial cells of the healthy nail bed than in the connective tissue. However, in the psoriatic nail bed and connective tissue IL-1α was absent. Therefore, we suggest that this cytokine is not locally expressed in psoriatic nails.
Staining with IL-6 showed positive expression in epithelia and connective tissue in psoriatic nails with predominance in the nail bed of our study group patients, while in the control group it was weakly expressed in the epithelia only. Higher IL-6 levels were observed in psoriatic lesions compared to non-lesional and normal healthy skin [18, 23, 7]. Therefore, we speculate that IL-6 similarly as in skin is an important cytokine in psoriatic nail disease due to the stimulation of other cytokine expression [18, 23, 7].
We found positive IL-10 expression both in epithelia and in connective tissue in the control group, prevailing in nail bed. However, we found even more pronounced IL-10 expression in epithelia of the nail bed in psoriatic nails. It was weakly expressed in the connective tissue of the psoriatic nails in some samples. We speculate that it indicates some significant differences in psoriatic nails in comparison to psoriatic skin plaques reflecting the unique pathways in psoriatic nail disease. Low levels of IL-10 in both psoriatic skin and in blister fluid were demonstrated by other authors [24, 14]. A total lack of IL-10R expression on keratinocytes in psoriatic lesions has been described [1, 5, 12, 17, 28, 30, 26].
Our findings provide perception of the site-specific inflammation of psoriatic nails where both elevations of antimicrobial peptides of innate immunity, common pro-inflammatory cytokine IL-6, as well as anti-inflammatory IL-10 can be observed.
An improvement in understanding the human nail immune system could ensure better comprehension of psoriatic nail disease and promote development of more targeted treatment options.
Conclusions
From anti-microbial peptides of defensin group, hβD-2 and hβD-3, but not hβD-4, are characteristic for human nail apparatus. The increased expression of these defensins in the psoriatic nail highlights the potential role of hβD-2 and hβD-3 for the innate immunity involvement in the pathogenesis of nail psoriasis.
In psoriatic nails, the pro-inflammatory (IL-1α) cytokine expression is decreased, but expression of IL-6 as well as the anti-inflammatory cytokine IL-10 is increased indicating stimulation of nail growth and increase of anti-inflammatory immunity.
Acknowlegements
Special thanks to Olga Zaikovska for input in providing tissue samples.
References
- Ameglio F., Bonifati C., Fazio M., et al. Interleukin-11 production is increased in organ cultures of lesional skin of patients with active plaque-type psoriasis as compared with nonlesional and normal skin. Similarity to interleukin-1 beta, interleukin-6 and interleukin-8 // Arch Dermatol Res, 1997; 289: 399–403.
- Asadullah K., Docke W. D., Ebeling M., et al. Interleukin 10 treatment of psoriasis: Clinical results of a phase 2 trial // Arch Dermatol, 1999; 135: 187–92.
- Asadullah K., Sterry W., Stephanek K., et al. IL-10 is a key cytokine in psoriasis // J Clin Incest, 1998; 101: 783–794.
- Büchau A. S., Gallo R. L. Innate immunity and antimicrobial defense systems in psoriasis // Clin Dermatol, 2007; 25: 616–624.
- Chang E. Y., Hammerberg C., Fisher G., et al. T-cell activation is potentiated by cytokines released by lesional psoriatic, but not normal, epidermis // Arch Dermatol, 1992; 128: 1479–1485.
- Christoph T., Müller-Rover S., Audring H., et al. The human hair immune system: Cellular composition and immune privilege // Br J Dermatol, 2000; 142: 862–873.
- De Jong E. M. G. J., Seegers B. A. M. P. A., Gulinck M. K., et al. Psoriasis of the nails associated with disability in a large number of patients: Results of a recent interview with 1728 patients // Dermatology, 1996; 193: 300–303.
- McGonaglea D., Benjaminb M., Ai Lyn T. The pathogenesis of psoriatic arthritis and associated nail disease: Not autoimmune after all // Current Opinion in Rheumatology, 2009; 21: 340–347.
- Dorschner R., Lopez-Garcia B., Massie J., Kim C., Gallo R. L. Innate immune defense of the nail unit by antimicrobial peptides // J Am Acad Dermatol, 2004; 50 (3): 343–348.
- Friedrich M., Docke W. D., Klein A., et al. Immunomodulation by interleukin-10 therapy decreases the incidence of relapse and prolongs the relapse-free interval in psoriasis // J Invest Dermatol, 2002; 118: 672–677.
- Gambichler T., Skrygan M., Tomi N. S., et al. Differential mRNA expression of antimicrobial peptides and proteins in atopic dermatitis as compared to psoriasis vulgaris and healthy skin // Int Arch Allergy Immunol, 2008; 147: 17–24.
- Gibbs A., Markham T., Walsh C., et al. Anakinra (Kineret) in psoriasis and psoriatic arthritis: A single-center, openlabel, pilot study // Arthritis Res Ther, 2005; 7: 68.
- Grossman R. M., Krueger J., Yourish D., et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes // Proc Natl Acad Sci USA, 1989; 86: 6367–6371.
- Harder J., Schröder J. M. Psoriatic scales: A promising source for the isolation of human skin-derived antimicrobial proteins // J Leukoc Biol, 2005; 77: 476–486.
- Ito, et al. Immunology of the human nail apparatus: The nail matrix is a site of relative immune privilege // J Invest Dermatol, 2005; 125: 1139–1148.
- Jinquan T., Vorum H., Larsen C. G., et al. Psoriasin: A novel chemotactic protein // J Invest Dermatol, 1996; 107: 5–10.
- Lande R., et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide // Nature, 2007; 449: 564–569.
- Longley B. J., Scher K. Diseases of the nails // Pathology of the skin with clinical correlation. – Ed. by McKee P. H., Calonje E., Granter S. R. – Philadelphia: Elsevier; 1996.
- McInnes I. B., Illei G. G., Danning C. L., et al. IL-10 improves skin disease and modulates endothelial activation and leukocyte effector function in patients with psoriatic arthritis // J Immunol, 2001; 167: 4075–4082.
- Mizutani H., Ohmoto Y., Mizutani T., et al. Role of increased production of monocytes TNF-α, IL-1 β and IL-6 in psoriasis: Relation to focal infection, disease activity and responses to treatments // J Dermatol Sci, 1997; 14: 145–153.
- Mussi A., Bonifati C., Carducci M., et al. IL-10 levels are decreased in psoriatic lesional skin as compared to the psoriatic lesion-free and normal skin suction blister fluids // J Biol Regul Homeost Agents, 1994; 8: 117–120.
- Nakatsuji T., Gallo R. L. Antimicrobial peptides: Old molecules with new ideas // Journal of Investigative Dermatology, 2012; 132: 887–895.
- Nickoloff B. J., Fiveson D. P., Kunkel S. L., Strieter R. M., et al. Keratynocyte interleukin-10 expression is upregulated in tape-stripped skin, poison ivy dermatitis and Sezary syndrome, but not in psoriatic plaques // Clin Immunol Immunopathol, 1994; 73: 63–68.
- Niyonsaba F., Nagaoka I., Ogawa H. Human defensins and cathelicidins in the skin: Beyond direct antimicrobial properties // Crit Rev Immunol, 2006; 26: 545–576.
- Okubo Y., Koga M. Peripheral blood monocytes in psoriatic patients overproduce cytokines // J Dermatol Sci, 1998; 17: 223–232.
- Paus R., Nickoloff B. J., Ito T. A “hairy” privilege // Trends Immunol, 2005; 26: 32–40.
- Pilmane M., Luts A., Sundler F. Changes in neuroendocrine elements in bronchial mucosa in adult diseased lung // Thorax, 1995; 50: 551–554.
- Reich A., Szepietowski J. C. Health-related quality of life in patients with nail disorders // Am J Clin Dermatol, 2011; 12: 313–320.
- Reich K., Bruck M., Grafe A., Vente C., et al. Treatment of psoriasis with interleukin-10 // J Invest Dermatol, 1998; 111: 1235–1236.
- Reich K., Garbe C., Blaschke V., et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation // J Invest Dermatol, 2001; 116: 319–329.
- Tan A. L., McGonagle D. Imaging of seronegative spondyloarthritis // Best Pract Res Clin Rheumatol, 2008; 22: 1045–1059.
- Tzu J., Mamelak A. J., Sauder D. N. Current advancements in the treatment of psoriasis: Immunobiologic agents // Clin Applied Immunol Rev, 2006; 6: 99–130.