Analysis of Seroreactivity to Recombinant B. burgdorferi Antigens BBA65 and BBA73
Abstract
Because of remarkable heterogeneity of Borrelia burgdorferi, the identification and characterisation of possible antigens is essential for the improvement of current laboratory diagnostics for Lyme borreliosis and vaccine development. Several new B. burgdorferi outer surface proteins have been identified over the past decade in an effort to characterise immunodominant antigens and evaluate them as possible vaccine candidates. In the present study, two recently identified recombinant immunogens of the PFam54 family (BBA65 and BBA73) were tested for the serodiagnostic potential.
The results of this study indicate that these proteins did not show sufficient antigenic properties to be used as single antigens; however, these proteins could be used as additional markers in multiantigen cocktail-based diagnostic tests for Lyme borreliosis. The detected antigenic properties of studied proteins indicate their possible involvement in the pathogenesis of LB, therefore further investigation of the functions is needed.
Introduction
Lyme borreliosis (Lyme disease, LB) is the most common vector-borne disease in the temperate zone of Northern hemisphere and an important emerging zoonosis in Europe, North America and Far East countries. LB is caused by the spirochetes of Borrelia burgdorferi sensu lato (B. burgdorferi s. l.) complex that is maintained in nature in enzootic cycles involving ticks of the Ixodidae family, as well as a range of mammalian and avian hosts [Piesman, 2004]. The disease is transmitted to humans after a bite of an infected Ixodes tick. LB usually begins with a slowly expanding skin lesion, called erythema migrans (EM) at the tick-bite site, and followed within days or weeks by disseminated infection if left untreated [Steere, 2004]. The disseminated borrelial infection may manifest with a wide range of clinical signs such as multiple EM skin lesions, borrelial lymphocytoma, nervous system involvement (neuroborreliosis), and arthritis. Cardiac manifestations were reported as well [Strle, 2009]. Because of the diversity of clinical symptoms, LB is often considered as a differential diagnosis [Wilske, 2007]. LB diagnosis is based primarily on clinical presentation and an assessment of tick-exposure risk. Serological two-tier testing is supporting diagnostic measure to confirm the diagnosis, in which the first tier is usually a sensitive enzyme linked immunosorbent assay (ELISA) and the second is a confirmatory Western blot [Stanek, 2012]. However, the limitations of antibody tests must be appreciated. Weak or absent antibody response in early LB, false positive results caused by the over-reading of the IgM immunoblots, and high background rates of seropositivity may confound the interpretation of seroreactivity. Beside this, the heterogeneity of the causative agent must be considered [Wilske, 2007]. Recently, in the study of Ang et al. it was shown that the assays used to detect anti-Borrelia antibodies have widely divergent sensitivity and specificity, and the choice of ELISA-immunoblot combination severely influences the number of positive results [Ang, 2011]. Thus, identifying and testing of novel target borrelia proteins for antigenicity is still in progress and offers a possibility to improve laboratory testing of LB.
One of the strategies to seek for a new potential serodiagnostic markers is to look for the Borrelia surface proteins that are markedly expressed during mammal infection. Similarly, surface protein expression increase in mammals comparing to tick environment could be interesting to characterise. Many studies have shown the differential gene expression regulated by changes in environmental conditions, the most studied of these cues is temperature shift. A large number of B. burgdorferi ORFs with significantly higher expression at 35 °C relative to 23 °C have been identified by global gene expression profiling study [Ojaimi, 2003]. In further studies, several outer surface exposed lipoproteins encoded by these genes have been identified that are actively expressed and/or immunogenic during Borrelia infections making them possible antigenic markers and vaccine candidates for Lyme disease [Adusumilli, 2010; Brooks, 2006]. Genes belonging to the paralogous gene family 54 were shown to be the most highly regulated group; moreover, members of this group, namely proteins BBA65, BBA66, BBA71 and BBA73 were detectable only in infectious B. burgdorferi B31 isolates [Hughes, 2008].
Aim
The aim of this study was to evaluate the possible use of two outer surface exposed B. burgdorferi proteins that are members of the paralogous gene family 54 (BBA65 and BBA73) as a serodiagnostic agents for LB diagnosis.
Material and methods
Human serum samples. A collection of human sera samples from patients who were diagnosed with LB and healthy control sera samples from healthy individuals without evidence of a current LB infection were used in this study. The study was approved by the LU EKMI (Institute of Experimental and Clinical Medicine, University of Latvia (Latvian: Latvijas Universitātes Eksperimentālās un klīniskās medicīnas institūts) ethical committee (26.05.2014). All samples were tested for LB by enzyme immunoassay for the in vitro diagnostic (Borrelia IgG + VlsE ELISA and Borrelia 14 kDa + OspC IgM ELISA, IBL International, Germany, data not shown). Based on these tests, LB sera samples were divided in three groups: IgM positive and IgG negative samples (early stage of disease, 14 samples), IgM positive and IgG positive samples (acute stage, 8 samples) and IgM negative, IgG positive samples (late stage, 13 samples). 10 LB IgM and IgG negative sera samples were included in the control sera group.
Cloning and expression of recombinant proteins. Genes coding for target proteins (BBA65 and BBA73) were amplified from B. burgdorferi s. l. strain B31 excluding the coding sequence for the hydrophobic region of N-terminal signal sequence (residues 1–24 for BBA65 and residues 1–27 for BBA73). Amplified genes were cloned in pETm_11 expression vector with integrated 6xHis tag and TEV protease cleavage site (EMBL, Heidelberg, Germany) and plasmids were transformed in competent E. coli RR1 cells by standard technique. The recombinant plasmids were isolated from positive clones by Plasmid Miniprep kit (Fermentas, Lithuania), and correct constructs were verified by sequencing. The N-terminal 6xHis tagged recombinant proteins were expressed in E. coli BL21 (DE3) by standard techniques. Briefly, the plasmid of the correct construct was transformed into E. coli cells and obtained transformants were grown overnight on LB agar plates containing kanamycin (10 mg/ml) at 37 °C. Seed material was grown from individual clone overnight without additional aeration in 2 × TY media supplemented with kanamycin (10 mg/ml). For expression experiments, seed material was inoculated in modified 2 × TYP media (TY supplemented with kanamycin (10 mg/ml), glucose (4 g/l) and 133 mM phosphate buffer pH 7.4) and cultivated with vigorous aeration until OD600 0.8–1.0 was reached. The recombinant protein expression was induced by adding IPTG (Isopropyl β-D-1-thiogalactopyranoside, 0.2 mM, Sigma-Aldrich, USA). The expression of target protein was confirmed by SDS-PAGE and Western blot analysis of total protein samples with Penta His antibody (Qiagen, Germany) followed by incubation with horseradish peroxidaselabelled anti-mouse antibody (ECL Anti-Mouse IgG, Horseradish Peroxidase-linked, GE Healthcare, UK). The colour was developed using 3.3-diaminobenzidine (SERVA, Germany).
Purification of recombinant proteins. The cells were harvested by centrifugation and lysed by sonication. The cell debris was removed by centrifugation and the recombinant proteins with a six-histidine tag were purified from the lysate using affinity chromatography with Ni-NTA agarose (Qiagen, Germany) column following the manufacturer’s instructions. The recombinant proteins were eluted with a high imidazole concentration. Buffer exchange was performed for eluted proteins into 20mM Tris-HCl pH 8.0 using Amicon centrifugal filter units (Millipore, UK). As a next purification step ionexchange chromatography on a Mono Q 10/100 GL column (GE Healthcare, UK) was performed followed by 6-histidine tag cleavage by TEV protease was carried out to obtain a pure protein. Protein samples were analysed by SDS-PAGE and the gels were stained with Coomassie brilliant blue R-250.
Western blot analysis. A Western blot assay was used to evaluate the possible antigenic property of the recombinant proteins obtained in this study. Two LB negative and two LB positive (acute stage) sera samples were used. The analysis was performed by standard techniques. Briefly, purified proteins were separated using polyacrylamide gel electrophoresis (PAGE) and transferred to Hybond™C Extra nitrocellulose membrane (GE Healthcare, UK). Non-specific binding sites were blocked by 5% non-fat dried milk in phosphate-buffered saline (PBS) for one hour at room temperature. The membrane was briefly rinsed in diluent and wash buffer (PBST, 0.1% Tween 20 in PBS) and incubated with human sera samples diluted 1 : 500 in PBST for an hour at room temperature. After rinsing in two changes of wash buffer, the membrane was incubated in anti-human IgM or IgG peroxidase-labelled antibodies diluted 1 : 10000 in PBST (Goat Anti-Human IgG (HRP); Goat Anti-Human IgM (HRP), Abcam, UK) for an hour at room temperature. After rinsing in two changes of wash buffer, the colour was developed using 3.3-diaminobenzidine (SERVA, Germany). Both recombinant proteins were probed by one sera sample simultaneously.
Solid-phase binding assays (ELISA). Microtitre wells (Maxisorb 96-well plates, Nunc, Thermo Fisher Scientific, Germany) were coated overnight at 4 °C with 100 μl of purified recombinant protein diluted in coating buffer (100 pg total protein). Wells were blocked overnight at 4 °C with 5% non-fat dried milk in PBS (blocking buffer). After washing 3 times with PBST human sera samples diluted 1 : 100 in blocking buffer were added to wells and incubated for 2 hours at room temperature. After washing 3 times with PBST, wells were incubated with horseradish peroxidase-labelled anti-human IgM or anti-human IgG antibody diluted 1 : 20 000 in blocking buffer (Goat Anti-Human IgM (HRP); Goat Anti- Human IgG (HRP), Abcam, UK) for 1 hour at room temperature. Wells were again washed four times with PBST, and the colour was developed using tetramethylbenzidine (Sigma-Aldrich, USA). Absorbance was read at 450 nm using Multilabel Counter 1420 (Perkin Elmer, USA). The mean optical density (OD) value for the control sera plus three Standard deviations (SD) was considered the cut-off value to determine positivity in each sample. Two independent experiments were performed.
Statistical analyses. Statistical analysis was performed using MedCalc (MedCalc Software, Belgium). In ELISA, a serum sample was considered positive if it reacted positively in two parallel tests for LB samples.
Results
Expression and purification of B. burgdorferi recombinant proteins. The selected outer surface lipoproteins of B. burgdorferi contained signal peptide at the N-terminal part of a protein with the average length of 20 amino acids as predicted by SignalP 3.0. [Bendtsen, 2004]. Therefore, the lipoproteins were expressed in E. coli as truncated constructs lacking the hydrophobic signal peptide. A sufficient expression of His-tagged soluble recombinant proteins in E. coli was followed by the downstream purification procedures. The combination of Ni-NTA affinity chromatography and ion-exchange chromatography was used and the purity of the isolated proteins as estimated from the Coomassie blue staining of the SDS-PAGE gels was at least 90% (Figure 1).
Figure 1. Testing of the seroreactivity of LB sera samples by Western blot
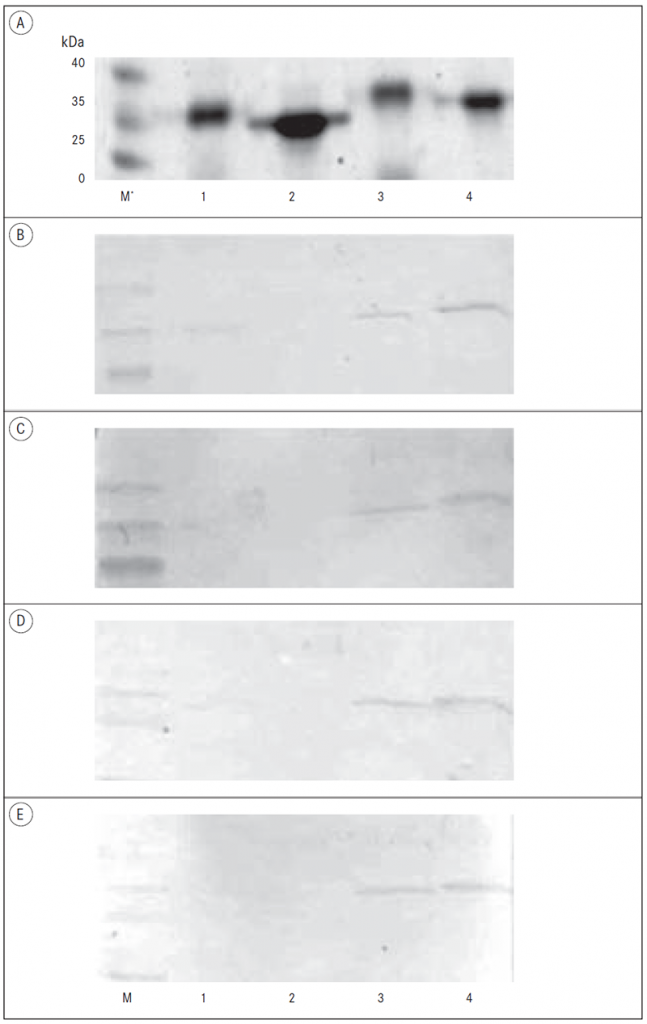
A – PAAG analysis of recombinant proteins;
B – Western blot of LB negative sample, IgG antibodies;
C – Western blot of LB negative sample, IgM antibodies;
D – Western blot of LB positive sample, IgG antibodies;
E – Western blot of LB positive sample, IgM antibodies.
* M – Protein weight marker; 1 – BBA65 with 6-histidine tag; 2 – BBA65 without 6-histidine tag; 3 – BBA73 with 6-histidine tag; 4 – BBA73 without 6-histidine tag.
Western blot analysis of BBA65 and BBA73 antigens. The antigenic properties of two recombinant proteins of the paralogous gene family 54 (BBA65 and BBA73) were tested by immunoblot analysis as described above by using LB positive and negative sera samples. Both antigens were tested simultaneously by individual sera sample in order to avoid the possible variability of Western blot conditions protein to protein. The results showed that BBA65 and BBA73 antigens were not able to distinguish LB positive and negative samples under the conditions used (Figure 1). There was not a detectable antibody response against recombinant BBA65 protein by immunoblot assay in patients with LB. By contrast, BBA73 showed positive response with LB positive as well as LB negative samples indicating lack of specificity for this antigen in this study under the conditions used.
Diagnostic effectiveness of BBA65 and BBA73 antigens. To evaluate the overall diagnostic effectiveness of BBA65 and BBA73 antigens for different stages of LB in human patients the quantitative ELISA was used. The immobilised antigens were probed with LB-positive sera samples, and both IgM and IgG antibodies were detected. A set of 10 sera samples from healthy individuals was used to define the cut-off value in each experiment. Samples with an absorbance higher than the cut-off value in two tests were considered positive. BBA65 antigen had an IgM antibody response only in case of early LB when 14.3 % of samples were positive (Table 1). By contrast, several samples representing all three stages of LB had IgG antibodies against the BBA65 protein: 14.3 %, 12.5 % and 15.4 % samples of early, acute and late LB stage were positive, respectively. Further, IgM and IgG antibodies against the BBA73 protein were detected. Similarly to BBA65 antigen, several samples representing all three stages of LB had IgG antibodies against the BBA73 protein: 7.1 %, 12.5 % and 15.4 % samples of early, acute and late LB stage were positive, respectively (Table 1). In addition, BBA73 antigen had IgM antibody response in case of early and late LB when 21.4 % and 7.7 % of samples were positive, respectively. None sera samples of acute LB phase had IgM antibodies against BBA73 protein.
In total, the results show that of 35 Latvian patients with LB, 5 (14.3 %) and 4 (11.4 %) had positive IgG antibody response to BBA65 and BBA73 antigen, respectively (Table 1). For IgM antibodies, 5.7 % (2 of 35) and 11.4 % (4 of 35) of LB samples were positive in case of BBA65 and BBA73 antigen, respectively.
Table 1. Testing of the seroreactivity of LB sera samples by ELISA
| Stage of disease | Number of samples, n | Positive sera samples, n (%) | |||
|---|---|---|---|---|---|
| BBA65 | BBA73 | ||||
| IgM | IgG | IgM | IgG | ||
| Early | 14 | 2 (14.3) | 2 (14.3) | 3 (21.4) | 1 (7.1) |
| Acute | 8 | 0 | 1 (12.5) | 0 | 1 (12.5) |
| Late | 13 | 0 | 2 (15.4) | 1 (7.70) | 2 (15.4) |
| Total | 35 | 2 (5.70) | 5 (14.3) | 4 (11.40) | 2 (15.4) |
Discussion
The identification of novel antigens has a range of potential applications for the improvement of the current laboratory diagnosis of LB and in the area of vaccine development. In the present study, we evaluate the possible antigenicity of two recombinant Borrelia proteins of paralogous gene family 54 – BBA65 and BBA73 in human patients with LB. It is known that many members of paralogous family 54 are affected by changing culture conditions, and several proteins of this family were shown to have immunogenic properties during murine and human infection [Angel, 2010; Barbour, 2008; Gilmore, 2007; Gilmore, 2008; Hughes, 2008; Ojaimi, 2003; Ouyang, 2008; Revel, 2002]. BBA65 and BBA73 are outermembrane localised proteins of B. burgdorferi. It was shown that antibodies specific for these proteins are detectable over the course of persistent infection in mice [Gilmore, 2007; Hughes, 2008]. However, there is little information about the presence of anti-family 54 proteins antibodies in sera of LB patients. Serologic analysis of Borrelia proteins in humans revealed a limited set of immunogens of this protein family. Little reactivity with another family 54 members, BBA64 and BBA66 was observed with either early-disseminated or late Lyme sera pools [Nowalk, 2006]. Similar results for these two antigens were obtained in the study of Barbour et al [Barbour, 2008]. Our results indicated that two members of paralogous gene family 54 – BBA65 and BBA73 – did not show sufficient antigenic properties in Latvian patients with LB to be used as single antigens in serodiagnostic tests. In Western blots analysis, there was not a detectable antibody response against recombinant BBA65 protein in patients with LB under the conditions used. By contrast, BBA73 showed the lack of specificity for this antigen in this study. The estimation of possible diagnostic effectiveness of BBA65 and BBA73 antigens by ELISA shows that 14.3 % and 11.4 % LB sera samples had positive IgG antibody response to BBA65 and BBA73 antigen, respectively. Importantly, the antibodies against these antigens were detected in sera samples representing all three stages of LB. By contrast, only 14.3 % of early LB samples were positive for BBA65 antigen. 21.4 % and 7.7 % of samples of early and late LB were positive for BBA73 protein, respectively. It is obviously, that paralogous could possess similar biochemical properties but distinct functional implications; however, it seems that temporal expression profile of many paralogous gene family 54 proteins inconveniences their usage as possible serodiagnostic markers for LB. In addition, the results indicated that due to the remarkable variation of antigenic profile, an optimal multi-antigen cocktail could be more effective to cover the heterogeneity of antibody responses and thus achieve the highest possible serodiagnosis test sensitivity and specificity.
Conclusions
Two studied proteins of paralogous gene family 54 – BBA65 and BBA73 – could be used as additional markers in multi-antigen cocktail-based diagnostic tests for Lyme borreliosis due to the remarkable variation of antigenic profile. The detected antigenic properties of studied proteins indicate their possible involvement in the pathogenesis of Lyme borreliosis, therefore further investigation of the functions is needed.
Acknowledgements
This work was supported by a grant of the Rīga Stradiņš University, Nr. RSU ZP 02/2013, and by grant of Latvian Council of Science, Nr. 10.0029.3.
We acknowledge Genome Database of Latvian Population, Latvian Biomedical Research and Study Centre for providing sera samples.
References
- Adusumilli S., Booth C. J., Anguita J., Fikrig E. Passage through Ixodes scapularis ticks enhances the virulence of a weakly pathogenic isolate of Borrelia burgdorferi // Infect Immun, 2009; 78: 138–44.
- Ang C. W., Notermans D. W., Hommes M., et al. Large differences between test strategies for the detection of anti-Borrelia antibodies are revealed by comparing eight ELISAs and five immunoblots // Eur J Clin Microbiol Infect Dis, 2011; 30: 1027–1032.
- Angel T. E., Luft B. J., Yang X., et al. Proteome analysis of Borrelia burgdorferi response to environmental change // PLoS One, 2010; 5 (11): e13800.
- Barbour A. G., Jasinskas A., Kayala M. A., et al. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi // Infect Immun, 2008; 76 (8): 3374–3389.
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. Improved prediction of signal peptides: SignalP 3.0. // J Mol Biol, 2004; 340: 783–795.
- Brooks C. S., Vuppala S. R., Jett A. M., Akins D. R. Identification of Borrelia burgdorferi outer surface proteins. // Infect Immun, 2006; 74: 296–304.
- Gilmore R. D. Jr., Howison R. R., Schmit V. L., et al. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice // Infect Immun, 2007; 75 (6): 2753–2764.
- Gilmore R. D. Jr., Howison R. R., Schmit V. L., Carroll J. A. Borrelia burgdorferi expression of the bba64, bba65, bba66, and bba73 genes in tissues during persistent infection in mice // Microb Pathog, 2008; 45 (5–6): 355–360.
- Hughes J. L., Nolder C. L., Nowalk A. J., et al. Borrelia burgdorferi surface-localized proteins expressed during persistent murine infection are conserved among diverse Borrelia spp //Infect Immun, 2008; 76: 2498–2511.
- Nowalk A. J., Gilmore R. D. Jr, Carroll J. A. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins // Infect Immun, 2006; 74 (7): 3864–3873.
- Ojaimi C., Brooks C., Casjens S., et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays // Infect Immun, 2003; 71: 1689–1705.
- Ouyang Z., Blevins J. S., Norgard M. V. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi // Microbiology, 2008; 154: 2641–2658.
- Piesman J., Gern L. Lyme borreliosis in Europe and North America // Parasitology, 2004; 129 Suppl: S191–220.
- Revel A. T., Talaat A. M., Norgard M. V. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete // Proc Natl Acad Sci USA, 2002; 99: 1562–1567.
- Stanek G., Wormser G. P., Gray J., Strle F. Lyme borreliosis // Lancet, 2012; 379: 461–473.
- Steere A. C. Lyme disease // N Engl J Med, 2001; 345: 115–125.
- Steere A. C., Coburn J., Glickstein L. The emergence of Lyme disease // J Clin Invest, 2004; 113: 1093–1101.
- Strle F., Stanek G. Clinical manifestations and diagnosis of Lyme borreliosis // Curr Probl Dermatol, 2009; 37: 51–110.
- Wilske B., Fingerle V., Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis // FEMS Immunol Med Microbiol, 2007; 49: 1.



