Prospective Evaluation of Dexmedetomidine Sedation during Regional Anaesthesia and Correlation with Electroencephalogram Index and Clinical Effects
Abstract
To evaluate dexmedetomidine sedation during regional anaesthesia and correlation with electroencephalogram index, clinical effects and the time and quality after awakening.
Prospective study involved 32 ASA I–II patients undergoing reconstructive surgeries and sedation with dexmedetomidine during regional anaesthesia. The loading dose of dexmedetomidine was 1 μg/kg over 10 min followed by infusion of 0.1–0.6 μg/kg/h. Depth of sedation was measured with electroencephalogram index. Time and quality of awakening were evaluated.
24/32 patients had a plexus brachialis block for hand reconstructive surgeries, 5/32 had spinal anaesthesia (SA) for leg reconstructive surgeries, 3/32 – SA and plexus brachialis block for free flap microvascular surgery. The mean duration of surgery was 89.38 ± 67.46 min, sedation – 102.81 ± 67.52 min.
After dexmedetomidine loading dose HR decreased by 8.44 ± 7.16 ×/min (p = 0.000), SBP decreased by 7.31 ± 12.03 mmHg (p = 0.002), DBP decreased by 4.75 ± 7.15 mmHg (p = 0.001). 2/32 patients had a bradycardia below 50 ×/min requiring a single dose of atropine, 5/32 had a temporary bradycardia that does not require treatment and in 25/32 cases sedation did not cause bradycardia. After loading dose SpO2 decreased by 1.28 ± 2.37 % (p = 0.005) and all patients had adequate spontaneous breathing without the need to use assisted ventilation or any airway device during sedation.
At the end of surgery all patients were promptly arousable with verbal stimulation without impaired cognitive abilities and psychomotor functions.
Loading dose of dexmedetomidine of 1 μg/kg intravenously over 10 min and a continuous infusion of 0.1–0.6 μg/kg/h provides safe management of sedation during regional anaesthesia under reconstructive surgery and correlates with electroencephalogram index of 50–70, provides fast and good quality of awakening and ensures a high patient satisfaction rate of sleep quality.
Introduction
Results of surgery under regional anaesthesia can be affected by fear and anxiety of a patient and discomfort from lying on the operating table [9]. In order to reduce a patient’s stress of being awake during the surgery under regional anaesthesia and to increase surgeon’s and anaesthetist’s satisfaction of the surgery, sedation is widely used [5, 9, 15, 17]. For surgeries under regional anaesthesia midazolam and propofol are the most common sedatives [2, 11, 17].
Dexmedetomidine is a selective α-2 receptor agonist with an anxiolytic, sedative and analgesic effect, and is not associated with respiratory depression [1, 2, 11, 17, 21, 25]. Compared to sedatives we have used so far, dexmedetomidine causes the “natural sleep” through inhibition of neuronal firing in the locus coeruleus in the brain stem which means a patient is easily arousable on verbal stimulation without impaired cognitive abilities and psychomotor functions [1, 7, 8, 16, 17, 18]. Dose dependent bradycardia and hypotension are the most frequently reported adverse reactions of dexmedetomidine [4, 6, 11, 17, 18, 21, 25].
Aim
The aim of this study was to evaluate dexmedetomidine sedation during regional anaesthesia and correlation with electroencephalogram index, clinical effects and time and quality after awakening.
Material and Methods
32 ASA I–II patients over the age of 18 scheduled for reconstructive surgeries under regional anaesthesia (RA) with dexmedetomidine sedation were enrolled in a prospective study, after an ethical committee’s approval and receiving written consent from all patients.
The following exclusion criteria were used:
- second or third degree heart block;
- bradycardia and arrhythmia;
- uncontrolled hypotension;
- mechanical ventilation;
- history of sleep apnea;
- liver failure;
- acute cerebrovascular accident;
- psychiatric disorder or currently being on psychotropic medication;
- pregnancy;
- coagulation disorders.
Anaesthesia methods. All patients received premedication with midazolam 7.5 mg before surgery.
For axillary brachial plexus blockade 20 ml 0.5 % bupivacaine and 20 ml 1 % lidocaine was used for hand reconstructive surgeries. For spinal anaesthesia (SA) 4 ml 0.5 % levobupivacaine was used for leg reconstructive surgeries. Axillary brachial plexus blockade and spinal anaesthesia was done for free flap microvascular surgeries not exceeding the maximum recommended doses of total local anaesthetics.
Sedation method. After confirmation of successful regional anaesthesia, loading dose of dexmedetomidine 1 μg/kg over 10 min was administered IV followed by a continuous infusion of 0.1–0.6 μg/kg/h until the end of the surgery. The continuous infusion of dexmedetomidine was adapted by EEG index maintaining a definite target EEG index of 50–70 (complies with EEG stage C2–D1) providing an efficient sedation during surgery.
Monitoring. Standard monitoring was used – non-invasive systolic (SBP) and diastolic (DBP) blood pressure, heart rate (HR), respiratory rate (RR), peripheral oxygen saturation (SpO2).
The respiratory depression was defined as oxygen saturation < 90 % or RR under 12 breaths/min. HR < 50 beats/min for more than 5 minutes was considered to be bradycardia and patients received a solution of 0.5 mg atropine IV.
EEG monitoring with EEG monitor Narcotrend-Compact M was used during sedation. It reflects the depth of sedation by identifying brain waves and therefore regulates sedative dosage [3, 10]. After regional anaesthesia was performed, three self-adhesive disposable electrodes were placed on the forehead using electrode gel, the patient’s leads were connected with Narcotrend-Compact M monitor and EEG recording was started to monitor the depth of sedation or hypnotic status of the patient during sedation. The monitor automatically classified EEG stages on a scale from stage A (conscious) to stage F (very deep sedation), this division refers explicitly to a range of EEG indexes: EEG stage A – awake (EEG index 95–100); EEG stage B, C – light sedation (EEG index 65–94); EEG stage D – moderate sedation (EEG index 37–64); EEG stage E, F – deep sedation (EEG index < 36) [3, 13].
Richmond Agitation Sedation Scale (RASS) was used to measure the level of sedation of the patients before and after loading dose and every 20 minutes until the end of surgery. Sedation was considered too deep when RASS was −4 or −5 (Table 1) [19].
Table 1. Richmond Agitation Sedation Scale
| Score | Term | Description |
|---|---|---|
| +4 | Combative | Overtly combative or violent, immediate danger to staff |
| +3 | Very agitated | Pulls on or removes tubes or catheters or has aggressive behaviour towards staff |
| +2 | Agitated | Frequent non-purposeful movements |
| +1 | Restless | Anxious or apprehensive but movements not aggressive or vigorous |
| 0 | Alert and calm | — |
| −1 | Drowsy | Not fully alert, but has sustained (more than 10 seconds) awakening, with eye contact/eye opening to voice |
| −2 | Light sedation | Briefly (less than 10 seconds) awakens with eye contact to voice |
| −3 | Moderate sedation | Any movement (but no eye contact) to voice |
| −4 | Deep sedation | No response to voice, but any movement to physical stimulation |
| −5 | Unarousable | No response to voice or physical stimulation |
Time and quality of awakening were evaluated at the end of each surgery. In recovery room at 30 minutes patient’s satisfaction with the quality of sleep was assessed by the use of handed out questionnaires.
Statistical analysis was performed with Microsoft Excel 2010 and SPSS (Statistical package for social sciences) 20. Data were evaluated with ANOVA (Analysis of variance) and Student’s t-test. Results with p values of < 0.05 were considered statistically significant.
Results
Demographic data and surgical characteristics were similar in all patients (Table 2). Types of RA used: 24/32 had an axillary brachial plexus blockade (75.0 %) for reconstructive surgeries in hand, 5/32 had a spinal anaesthesia (15.6 %) for reconstructive surgeries in leg, 3/32 – SA with plexus blockade (9.4 %) for free flap microvascular surgery.
Mean HR during sedation was 62.86 ± 7.90 beats/min. After dexmedetomidine loading dose, mean HR decreased by 8.44 ± 7.16 beats/min (p = 0.000) (before loading dose 73.75 ± 10.34 beats/min) (Table 3). We observed bradycardia below 50 beats/min requiring a single minimum dose of atropine in 2/32 patients (6.3 %), 5/32 patients (15.6 %) had a temporary bradycardia that does not require treatment and in 25/32 cases (78.1 %) sedation with dexmedetomidine did not cause bradycardia (Figure 1). Loading dose bradycardia did not appear in any of the patients while EEG index was 20–80 (Figure 2).
Two patients with bradycardia requiring atropine had a Narcotrend EEG stage A – awake (EEG index of 95–100). Out of those two patients – one patient was a 28-year-old professional athlete, he had bradycardia during loading dose when EEG index was 98, the other patient was a 71-year-old man who had an acute surgery and bradycardia occurred 20 minutes after the start of continuous infusion at EEG index 96.
When comparing all types of RA used, HR was similar in all three RA groups after the loading dose (Figure 3).
Table 2. Demographic data and surgical characteristics
| Gender, female/male, n (%) | 14 (43.8 %) female | 18 (56.2 %) male |
|---|---|---|
| Age, years | 46.44 ± 16.88 (20 to 74) | |
| Weight, kg | 75.00 ± 14.11 (50 and 120) | |
| Height, cm | 172.85 ± 8.31 (163 and 185) | |
| Type of surgery | 28 (87.5 %) elective | 4 (12.5 %) acute |
| Duration of surgery, min | 89.38 ± 67.46 (minimum 20, maximum 300) | |
| Duration of sedation, min | 102.81 ± 67.52 (minimum 35, maximum 310) | |
Table 3. Changes in heart rate (HR) during sedation
| Heart rate | SM * | SSD ** | Number of patients, n |
|---|---|---|---|
| HR before loading dose, b/min | 73.75 | 10.336 | 32 |
| HR after loading dose, b/min | 65.31 | 9.107 | 32 |
| HR 10 min after SCI***, b/min | 63.78 | 6.568 | 32 |
| HR 20 min after SCI, b/min | 62.53 | 8.320 | 32 |
| HR 30 min after SCI, b/min | 61.39 | 7.013 | 31 |
| HR 40 min after SCI, b/min | 60.36 | 7.395 | 28 |
| HR 50 min after SCI, b/min | 59.62 | 7.180 | 21 |
| HR 60 min after SCI, b/min | 58.06 | 7.198 | 17 |
| HR 1.5 h after SCI, b/min | 58.33 | 8.818 | 9 |
| HR 2 h after SCI, b/min | 55.50 | 8.018 | 8 |
| HR at the end of surgery, b/min | 60.34 | 7.065 | 32 |
* SM – statistic mean;
** SSD – standard statistic deviation;
*** SCI – start of continuous infusion.
Figure 1. Incidence (%) of bradycardia during sedation
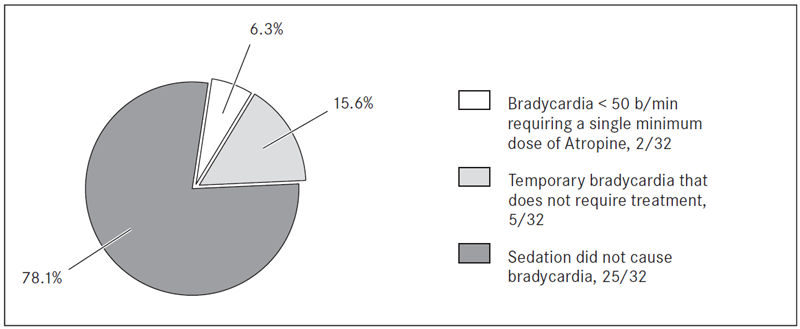
Figure 2. Changes of heart rate (HR) after loading dose according to electroencephalogram (EEG) index (20–80) *
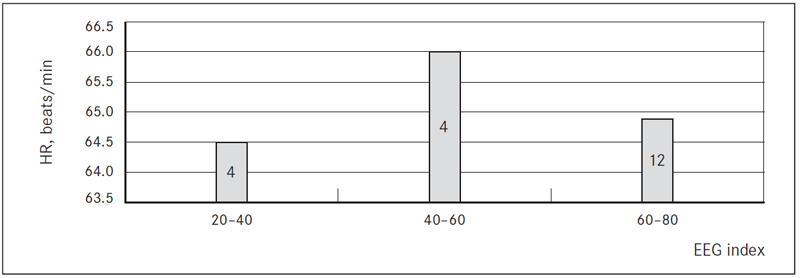
* The number printed on bars indicates the number of patients in current group of EEG index.
Figure 3. Changes of heart rate (HR) after loading dose according to types of regional anaesthesia (RA) used *
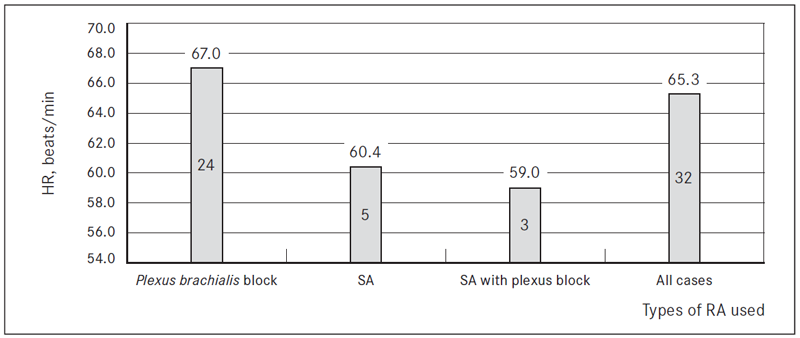
* The number printed on bars indicates the number of patients in current group of RA used and the mean value of HR.
We did not observe any significant changes in SBP and DBP after dexmedetomidine loading dose was administered – mean SBP decreased by 7.31 ± 12.03 mmHg (p = 0.002) and mean DBP decreased by 4.75 ± 7.15 mmHg (p = 0.001). Mean SBP during the sedation was 119.39 ± 15.16 mmHg, mean DBP was 71.99 ± 9.83 mmHg (Table 4). Results showed that sedation with dexmedetomidine caused neither of the following in any of the patients: hypotensia, the need to stop the continuous infusion or add other sedatives.
We observed minimal decrease in SpO2 levels after the loading dose (1.28 ± 2.37 %, p = 0.005) without the need to use assisted ventilation or any airway device. All patients had adequate spontaneous breathing during their sedation.
Mean EEG index after loading dose decreased by 19.72 ± 23.85 (p = 0.000) indicating light to moderate sedation (Figure 4). During dexmedetomidine sedation, mean EEG index was 68.53 ± 21.70 which was within the target EEG index rage. The target level of sedation was reached 10 minutes after the start of continuous infusion. The mean lowest recorded EEG index was 53.10 ± 25.00 after a continuous infusion of 30 minutes. The environment had a significant negative impact to the quality of dexmedetomidine sedation. Increased noise levels rose EEG index during surgery; therefore, patients woke up. However, those patients were able to quickly fall back asleep. According to RASS, the level of sedation during surgery was from 0 to −3. We observed that at the end of surgery, all patients were promptly arousable with verbal stimulation without impaired cognitive abilities and psychomotor functions. According to answers from their questionnaires all patients were satisfied with the sedation they received.
Table 4. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) values during sedation
| Systolic blood pressure | SM * | Number of patients, n |
|---|---|---|
| SBP before loading dose, mmHg | 136.06 | 32 |
| SBP after loading dose, mmHg | 128.75 | 32 |
| SBP 10 min after SCI **, mmHg | 123.22 | 32 |
| SBP 20 min after SCI, mmHg | 116.22 | 32 |
| SBP 30 min after SCI, mmHg | 114.68 | 31 |
| SBP 40 min after SCI, mmHg | 114.75 | 28 |
| SBP 50 min after SCI, mmHg | 110.62 | 21 |
| SBP 60 min after SCI, mmHg | 112.71 | 17 |
| SBP 1.5 h after SCI, mmHg | 112.44 | 9 |
| SBP 2 h after SCI, mmHg | 112.50 | 8 |
| SBP at the end of surgery, mmHg | 114.34 | 32 |
| Diastolic blood pressure | SM * | Number of patients, n |
| DBP before loading dose, mmHg | 82.75 | 32 |
| DBP after loading dose, mmHg | 78.00 | 32 |
| DBP 10 min after SCI **, mmHg | 73.94 | 32 |
| DBP 20 min after SCI, mmHg | 69.69 | 32 |
| DBP 30 min after SCI, mmHg | 68.23 | 31 |
| DBP 40 min after SCI, mmHg | 69.75 | 28 |
| DBP 50 min after SCI, mmHg | 66.00 | 21 |
| DBP 60 min after SCI, mmHg | 67.06 | 17 |
| DBP 1.5 h after SCI, mmHg | 68.33 | 9 |
| DBP 2 h after SCI, mmHg | 71.88 | 8 |
| DBP at the end of surgery, mmHg | 68.75 | 32 |
* SM – statistic mean;
** SCI – start of continuous infusion.
Figure 4. Changes of electroencephalogram (EEG) index before (A) and after (B) loading dose of dexmedetomidine
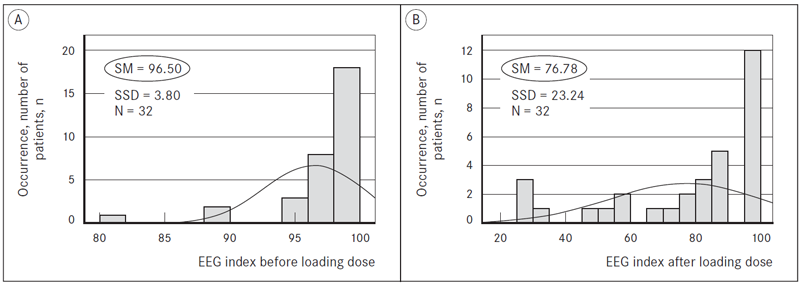
N – number of patients, n;
SM – statistic mean;
SSD – standard statistic deviation.
Discussion
Sedation is widely used to reduce anxiety and improve comfort during reconstructive surgery under regional anaesthesia [8]. A new sedative agent dexmedetomidine (highly selective α-2 adrenergic receptor agonist) has been used to provide sedation, analgesia, anxiolysis and to reduce opioid requirements. Because of dexmedetomidine properties to promote a sedative state similar to physiological sleep (stage 1 and 2 non-rapid eye movement sleep) without respiratory depression, it is described as an ideal sedative [8, 14]. Although, dose dependent bradycardia and hypotension are often reported, particularly with more rapid infusion and in patients with pre-existing cardiac problems [4, 6, 17, 20, 21]. We found that low doses of dexmedetomidine (loading dose 1 μg/kg/10 min, a continuous infusion 0.1–0.6 μg/kg/h) during reconstructive surgeries under RA sedation did not cause any significant haemodynamic instability and bradycardia was not seen while EEG index 20–80. Similar results were reported by Arain S. R. et al. and Kilic N. et al. using dexmedetomidine loading dose of 1 μg/kg over 10 minutes followed by a continuous infusion of 0.4–0.7 μg/kg/h and 0.2–0.7 μg/kg/h providing efficient sedation [10, 15]. Ok H. G. et al. study results showed that dexmedetomidine loading dose of 1 μg/kg over 10 minutes is sufficient for surgeries up to 60 minutes long. A continuous infusion of dexmedetomidine of 0.2 μg/kg/h is sufficient for surgeries up to 80 minutes long and a continuous infusion of 0.4 μg/kg/h provides efficient sedation for surgeries up to 120 minutes long under spinal anaesthesia [17].
The incidence of bradycardia and hypotension is the most frequently reported dexmedetomidine adverse haemodynamic response associated with increased dosage and concentration [4, 6, 11, 17, 21]. A study by Ok H. G. et al. reported that the frequency of bradycardia and hypotension does not increase when a low dose of dexmedetomidine is administered IV for sedation under spinal anaesthesia. In our study the incidence of bradycardia requiring atropine was low (2 out of 32 patients) in addition – hypotension was never recorded.
Authors report dexmedetomidine as a useful sedative for procedures because of its minimal effects on the respiratory system [15, 17]. Belleville J. P. et al. reported a study of examined ventilatory effects of a 2-minute intravenous four different dose level of dexmedetomidine infusion. Results showed that right after the maximum dose of 2.0 μg/kg irregular breathing with periods of apnea were noticed; however, there was no significant arterial oxygen desaturation below 90 % [22].
In this study the level of sedation was assessed by RASS (the level was from 0 to −3 during sedation) and the depth of sedation or hypnotic status was measured by Narcotrend EEG monitor maintaining the pre-set target level EEG index between 50 and 70. Jiang Y. et al. reported that the use of a Narcotrend monitor for monitoring the hypnotic status may guide sedation according to the EEG index and, therefore, the dose of sedatives is more precise [10]. It is necessary to emphasise that there are some limitations using assessment scales like Richmond Agitation Sedation Scale, Ramsay Sedation Scale or Observer’s Assessment of Alertness/Sedation scale. Assessment scales are subjective interpretations by the observers of patient’s alertness, the quality of sedation is compromised because the assessments require a patient to be awaken every time an assessment is done [17]. Therefore, authors recommend using Bispectral Index System (BIS) or Narcotrend EEG monitoring for measuring the depth of sedation instead of assessment scales [17, 24]. BIS and Narcotrend EEG monitoring provide real time assessment and quality of sedation is not compromised by external stimulation [24]. Ekin A. et al. in a study measuring depth of sedation with BIS reported that environmental stimuli and application of tourniquet increase values of BIS, although it does not affect a patient’s satisfaction with his sedation [5].
There are reports about dexmedetomidine’s advantages of providing fast recovery after procedures, patients are easily arousable on verbal stimulation and able to perform psychomotor testing without impaired cognitive abilities and psychomotor functions [1, 12, 23]. In our study, at the end of each surgery, all patients were promptly arousable with verbal stimulation.
Conclusion
- Loading dose of dexmedetomidine of 1 μg/kg administered intravenously over 10 minutes and a continuous infusion of 0.1–0.6 μg/kg/h provides safe management of sedation during regional anaesthesia under reconstructive surgery and correlates with electroencephalogram index of 50–70 and Richmond Agitation Sedation Scale 0 to −3.
- After loading dose of dexmedetomidine of 1 μg/kg administered intravenously over 10 minutes while electroencephalogram index was 20–80, significant clinical effects did not appear – bradycardia and respiratory instability, all patients had adequate spontaneous breathing without the need to use assisted ventilation or any airway device during sedation.
- The loading dose of dexmedetomidine of 1 μg/kg administered intravenously over 10 minutes and a continuous infusion of 0.1–0.6 μg/kg/h provides fast and good quality of awakening and ensures a high patient satisfaction rate of sleep quality during regional anaesthesia.
References
- Arain S. R., Ebert T. J. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesthesia & Analgesia, 2002; 95: 461–466.
- Barash P. G., Cullen B. F., Stoelting R. K., Cahalan M. K., et al. Clinical Anesthesia Fundamentals. Lippincott Williams and Wilkins, 2015, 155–163.
- Brown E. N., Solt K., Purdon P., Johnson-Akeju O. Monitoring brain state during general anesthesia and sedation. Miller’s Anesthesia / Ed. by Miller R. D. Vol 2, 8th ed. London: Elsevier Health Sciences, 2015, 1524–1530.
- Ebert T. J., Hall J. E., Barney J. A., Uhrich T. D., et al. The effect of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology, 2000; 93: 382–394.
- Ekin A., Donmez F., et al. Patient-controlled sedation in orthopedic surgery under regional anesthesia: a new approach in procedural sedation. Revista Brasileira de Anestesiologia, 2013; 63 (5): 410–414.
- Esmaoglu A., Yegenoglu F., Akin A., Turk C. Y. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesthesia & Analgesia, 2010; 111 (6): 1548–1551.
- Grewal A. Dexmedetomidine: new avenues. Journal of Anaesthesiology Clinical Pharmacology, 2011; 27 (3): 297–302.
- Ho K. M. Is dexmedetomidine an ideal sedative agent for neurosurgical patients? Anaesthesia and Intensive Care, 2012; 40 (6): 927.
- Hohener D., Blumenthal S., Borgeat A. Sedation and regional anaesthesia in the adult patient. British Journal of Anaesthesia, 2008; 100 (1): 8–16.
- Jiang Y., Qiao B., Wu L., Lin X. Application of Narcotrend Monitor for evaluation of depth of anesthesia in infants undergoing cardiac surgery: a prospective control study. Revista Brasileira de Anestesiologia, 2013; 63 (3): 273–278.
- Jun G. W., Kim M. S., Yang H. J., Sung T. Y., et al. Laparoscopic appendectomy under spinal anesthesia with dexmedetomidine infusion. Korean Journal of Anesthesiology, 2014; 67 (4): 246–251.
- Kilic N., Sahin S., Aksu H., Yavascaoglu B., et al. Conscious sedation for endoscopic retrograde cholangiopancreatography: dexmedetomidine versus midazolam. The Eurasian Journal of Medicine, 2011; 43: 13–17.
- Kreuer S., Wilhelm W. The Narcotrend Monitor. Best Practice and Research Clinical Anaesthesiology, 2006; 20: 111–119.
- Kunisawa T. Dexmedetomidine hydrochloride as a long-term sedative. Therapeutic and Clinical Risk Management, 2011; 7: 291–299.
- Mackenzie N. Sedation during regional anaesthesia: indications, advantages and methods. European Journal of Anaesthesiology, 1996; 13: 2–7.
- Nelson L. E., Lu J., Guo T., Saper C. B., Franks N. P., Maze M. The α2-adrenoreceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathways to exert its sedative effects. Anesthesiology, 2003; 98: 428–436.
- Ok H. G., Baek S. H., Baik S. W., Kim H. K., et al. Optimal dose of dexmedetomidine for sedation during spinal anesthesia. Korean Journal of Anesthesiology, 2013; 64 (5): 426–431.
- Reddy V. S., Shaik N. A., Donthu B., Reddy V. K., et al. Intravenous dexmedetomidine versus clonidine for prolongation of bupivacaine spinal anesthesia and analgesia: a randomized double-blind study. Journal of Anaesthesiology Clinical Pharmacology, 2013; 29 (3): 342–347.
- Sessler C. N., Gosnell M. S., Grap M. J., Brophy G. M., et al. The Richmond Agitation– Sedation Scale: validity and reliability in adult intensive care unit patients. American Journal of Respiratory and Critical Care Medicine, 2002; 166: 1338–1344.
- Shelly M. P. Dexmedetomidine: a real innovation or more of the same? British Journal of Anaesthesia, 2001; 87 (5): 677–678.
- Song J., Kim W. M., Lee S. H., Yoon M. H. Dexmedetomidine for sedation of patients undergoing elective surgery under regional anesthesia. Korean Journal of Anesthesiology, 2013; 65 (3): 203–208.
- Venn R. M., Hell J., Grounds R. M. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Critical Care, 2000; 4: 302–308.
- Venn R. M., Karol M. D., Grounds R. M. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. British Journal of Anaesthesia, 2002; 88 (5): 669–675.
- Wang T., Ge S., Xiong W., Zhou P., et al. Effects of different loading doses of dexmedetomidine on bispectral index under Stepwise Propofol Targer-Controlled Infusion. Pharmacology, 2013; 91: 1–6.
- Zhang X., Bai X. New therapeutic uses for an alpha2 adrenergic receptor agonist – Dexmedetomidine in pain management. Neuroscience Letters, 2014; 561: 7–12.



