Reduced Retinal Nerve Fibre Layer Prediction for Multiple Sclerosis Patients
Abstract
Multiple sclerosis often affects afferent visual system. Recently it has been recommended to introduce investigation with optical coherence tomography as a part of routine monitoring in multiple sclerosis patients. However, not in every multiple sclerosis centre optical coherence tomography technique is available. The aim of our study was to develop a statistical model for predicting the risk of reduced retinal nerve fibre layer using simple structural and functional visual system tests.
The study recruited 76 multiple sclerosis patients and 28 normal controls. Routine opthtalmologic and radiologic tests were performed. Independent variables, believed to be associated with reduced retinal nerve fibre layer, were included in the study. Forward binary logistic regression was used to find significant variables and probabitity of retinal nerve fibre layer reduction.
The changes in visual evoked potential amplitude and latency, the number of demyelinating lesions in magnetic resonanse investigation and disease duration are the independent variables found significant to predict the risk of reduced retinal nerve fibre layer (p < 0.05).
Forward stepwise (likelihood ratio) binary logistic regression model is an accurate method to predict reduced retinal nerve fibre layer in the presence of reduced N75 / P100 amplitude, prolonged P100 latency with the high number of demyelinating lesions in magnetic resonanse investigation. Our model may be applied for identification of individuals who are at a high risk of reduced retinal nerve fibre layer in order to refer them to optical coherence tomography examination.
Introduction
Afferent visual pathways are a suitable clinical model for investigation of multiple sclerosis (MS) and neuroprotective drugs (Costello, 2013). Significant advantage is that visual pathways are available for detailed and direct structural and functional studies. A recently published article suggests that OCT method should be considered as a part of routine monitoring of MS patients (Saidha and Calabresi, 2014). However, such an approach was later criticised and the usefulness of OCT questioned (Jenkins and Toosy, 2014). The fact should be taken into account that not in all MS centres OCT method is available to date. The question arises whether changes detectable by OCT examination could be estimated by using other visual system tests, regardless of the history of optic neuritis (ON) episode.
The aim of our study was to develop a statistical model for predicting the risk of reduced retinal nerve fibre layer (RNFL) using simple structural and functional visual system tests for everyday use.
Material and Methods
Subjects. The cross-sectional study included 76 relapsing-remitting MS patients both with and without ON history. The control group included 28 age-matched healthy individuals.
MS patients were recruited from Pauls Stradins Clinical University Hospital, Multiple Sclerosis Center within the period from October 2011 to April 2014.
Inclusion criteria:
- relapsing remitting MS diagnosis, based on the 2010 McDonald criteria;
- for MS patients with history of ON ≥ 6 months after the unilateral ON episode;
- ≥ 30 days after corticosteroid therapy.
Exclusion criteria:
- acute ON clinical signs;
- refraction disorders exceeding ± 6 diopters;
- other neurological and ophthalmic diseases, which can affect the afferent visual system;
- inability to participate in the visual system examinations.
Tests. Following functional and structural visual system tests were performed for each eye separately.
Visual acuity determination with visual acuity test figures using Snellen chart located 6 metres from the patient’s face was performed. Visual acuity measurement results were expressed as a decimal, recording the lowest line of the smaller figures, which the patient was able to name error-free. Whenever necessary, the corrected visual acuity was used.
The computerised visual field perimetry was performed. The 30-2 threshold programme was used, retinal sensitivity was measured at 54 points; the points tested were distributed 3° from the vertical or horizontal meridian. Each light stimulus was displayed for about 0.2 seconds, the test results were analysed using a decibel scale.
In order to inspect the colour vision, the Ishihara test was used, showing the polychromatic tables to a patient in daylight, from a distance of one metre, for five seconds. For patients who were unable to distinguish the hidden numbers or shapes, colour vision disorders were diagnosed.
For all of the study participants pattern-reversal VEP record was performed using the hardware “RETI port 21 ROLAND CONSULT”. Individuals were placed 70 cm away from the screen, fixing their view on the red dot in the centre of the screen. If necessary, a full refractive correction was made. In order to establish the potential, vision was repeatedly stimulated in a monocular way with a blackwhite video monitor at 1.6 Hz frequency. Record of the potentials was made with the disc electrodes, placing them in the brain visual cortical projection areas of the International 10–20 system (Odom, Bach et al., 2010). The average potentials of action were filtered and analysed, repeatedly performing 100 re-stimulations twice for each eye. N75 / P100 amplitude measurements in microvolts (mcV), as well as P100 latency measurements in miliseconds (ms) have been made. N75 / P100 amplitude below 10.52 mkV was considered to be reduced, P100 latency, longer than 110.25 ms, was considered to be prolonged.
The investigation of the frontal part of the eye was carried out using a slit-lamp biomicroscopy. Fundus oculi examination was performed using a 90 diopter lens. In the fundus oculi examination the following parameters were evaluated: colour of the optic nerve disc, borders, level, as well as the temporal pallor of the optic nerve disc was diagnosed.
With the OCT method (Heidelberg Engineering SPECTRALIS), RNFL thickness was measured in six standard sectors (temporal, temporal upper, temporal lower, nasal, nasal upper and nasal lower), measurements expressed in micrometres (mcm). RNFL thickness results were evaluated according to the OCT apparatus normative database, where green-marked areas were classified as normal, but the red-marked areas were considered abnormally reduced. OCT images of unsatisfactory quality were rejected.
MRI examination of the brain was performed by means of MRI device with a 1.5 T magnetic field strength. FLAIR 3D with MPR reconstructions axially and/or sagittally, coronary, T1 3D (T1 3D IR) with MPR reconstructions axially and/or sagittally, coronary were performed. T2 axially 4 mm and T2 FS for optic nerves coronary 3 mm including chiasma opticum was conducted. When assessing the MRI results, the number and localisation of demyelinating lesions and active, contrast enchancing lesion existence were analysed.
Statystical analysis. Statisical analysis was carried out by means of IBM SPSS v.22. programme. Data were presented as mean (M) and standard deviation (SD) or median (Me) and interquartile range (IQR) for continuous variables, and counts and percentages (%) for categorical variables. Logistic regression modeling techniques were used to determine which patient factors were associated with the binary outcome. All tests were two-sided and considered statistically significant at p < 0.05.
Results
The study included 76 MS patients whose average age was 38.64 years (SD = 10.60). The control group included 28 healthy subjects aged 19 to 65 years and in this group the mean age was 35.78 years (SD = 12.14). On the basis of the analysis of variance (ANOVA), it was found that MS patients and the control group by the average ages were not statistically significantly different (p = 0.12).
Comparing the control group and MS patients’ group through OCT analysis, it was concluded that RNFL set was statistically significantly different (p < 0.05) in all quadrants. While, on the basis of the ROC curve analysis, it was concluded that the biggest difference was detected in the temporal RNFL quadrant (accordingly AUC = 0.72; 95 % CI: 0.64 to 0.79; p < 0.01). Therefore, in the forthcoming calculations, data for the temporal quadrant of RNFL (RNFLT) were analysed.
Demographic details and clinical details of MS patients and controls are represented in Table 1.
Table 1. Clinical and demographical characteristic of subjects
| Characteristic | MS patients | Controls |
|---|---|---|
| Number | 76 | 28 |
| Age, years | 38.64 (SD = 10.60) | 35.78 (SD = 12.14) |
| Disease duration, months | 39.56 (6–384) | N/A |
| EDSS Me, IQR | 1.5 (1) | N/A |
| Visual acuity (corrected) | 0.94 (SD = 0.16) | 1.00 |
| Mean N75 / P100 amplitude, mcV | 9.69 (SD = 4.85) | 14.51 (SD = 3.35) |
| Mean P100 latency, ms | 118.53 (SD = 16.33) | 101.81 (SD = 5.66) |
| Mean RNFLT, mcm | 61.62 (SD = 14.33) | 70.93 (SD = 9.50) |
| Colour vision disturbances (eyes), % | 27 (17.8 %) | N/A |
| Visual field defects (eyes), % | 101 (66.4 %) | N/A |
Logistic regression model development. In total, assessing all the factors affecting RNFLT and basing on the logistic regression analysis method, the mathematical models were developed that further assisted in prediction of reduced RNFLT.
Out of the 11 independent variables, only four variables were found to be significant (p < 0.05) after applying binary logistic regression analysis to predict risk of reduced RNFLT.
A binary logistic regression analysis was computed to analyse the impact of those predictors on reduced RNFLT that proved to be significant in the univariate comparisons. Following regression models were developed.
The first model. In the first logistic regression model we placed the following parameters: reduced VEP N75 / P100 amplitude and prolonged P100 latency. In the model both the parameters and the constant were statistically significant (p < 0.05). Logistic regression equation coefficients and their credibility predicting reduced RNFLT are shown in Table 2.
Table 2. Predictors of the first regression model for classifying reduced RNFLT
| Predictors | Beta | SE | Wald | p-value | OR (95 % CI) |
|---|---|---|---|---|---|
| Prolonged P100 latency | 1.83 | 0.42 | 18.90 | < 0.001 | 6.23 (2.73–14.22) |
| Reduced N75 / P100 amplitude | 1.41 | 0.46 | 9.50 | 0.002 | 4.12 (1.67–10.16) |
The regression model explained 30 % of the variance (Nagelkerke’s R2 = 0.30) and altogether classified 79.8 % of the reduced RNFLT correctly.
The resulting logistic regression equation in the logit form is as follows:
Logit (for the reduced RNFLT) = – 2.53 + 1.31 × N75 / P100 amplitude + 2.36 × P100 latency.
In logistic regression model, we have computed the predicted risk (P) with the help of the following equation:

Analysing the odds ratios from the calculated logistic regression model, it was found that for individuals, whose N75 / P100 amplitude was reduced, the chance to have a reduced RNFLT was 4.12 (95 % CI: 1.67–10.16) times more than for those with normal amplitude. Conversely, for the individuals having prolonged P 100 latency, the chance to have a reduced RNFLT was 6.23 (95 % CI: 2.73 to 14.22) times more than for patients with normal VEP latency.
The second model. In the next model, which characterises a reduced RNFLT, in addition to changed VEP parameters we have added also the patients’ disease duration, and obtained the following picture (Table 3).
Table 3. Predictors of the second regression model for classifying reduced RNFLT
| Predictors | Beta | SE | Wald | p-value | OR (95 % CI) |
|---|---|---|---|---|---|
| Prolonged P100 latency | 0.68 | 0.43 | 14.90 | < 0.001 | 5.37 (2.28–12.63) |
| Reduced N75 / P100 amplitude | 1.65 | 0.50 | 10.96 | 0.001 | 5.23 (1.96–13.92) |
| Disease duration | 0.05 | 0.002 | 6.80 | 0.009 | 1.005 (1.001–1.01) |
The second logistic model classifies 76.3 % of the reduced RNFLT correctly and explains 35 % of the variance (Nagelkerke’s R2 = 0.35).
The resulting second logistic regression equation in the logit form is as follows:
Logit (for reduced RNFLT) = − 3.23 + 1.65 × N75 / P100 amplitude + 1.68 × P100 latency + 0.05 × disease duration
Conversely, the equation useful for practice is as follows:
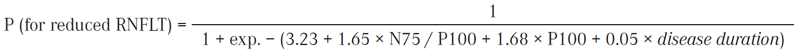
As can be seen from the second equation, the chance to get reduced RNFLT increases by 5% with each year of the disease.
The third model. The third model also involves changes in the fundus oculi, this parameter was with p = 0.74, which means that this parameter only could potentially have a statistically significant effect on reduced RNFLT existence. In this model, Nagelkerke’s R2 increases by 2.7 % to 37.4 %, and as we have concluded, for individuals who are observed changes in fundus oculi, the chance to get reduced RNFL is 2.39 (95 % CI: 0.92 to 6.25) times more than for patients with normal fundus oculi finding.
The fourth model. Logistic model, which has classified our patients in the best way in order to predict reduced RNFLT embodied prolonged P 100 latency, reduced N75 / P100 amplitude and the number of MRI demyelinating lesions (Table 4).
Table 4. Predictors of the third regression model for classifying reduced RNFLT
| Predictors | Beta | SE | Wald | p-value | OR (95 % CI) |
|---|---|---|---|---|---|
| Prolonged P100 latency | 1.70 | 0.59 | 8.21 | 0.01 | 5.523 (1.71–17.77) |
| Reduced N75 / P100 amplitude | 1.54 | 0.61 | 6.36 | 0.01 | 4.68 (1.41–15.54) |
| The total number of lesions in MRI | 0.06 | 0.02 | 8.15 | 0.01 | 1.07 (1.02–1.12) |
The fourth regression model has explained even 52 % of the variance (Nagelkerke’s R2 = 0.52) and classified 84.4 % of the reduced RNFLT correctly, which is a very high ratio.
Logit (for reduced RNFLT) = −4.01 + 1.54 × N75 / P100 amplitude + 1.70 × P100 latency + 0.06 × number of foci.
From the equation, it is clear that log odds have linear relationship. The predicted risk of RNFLT can be calculated by means of
![]()
By increasing the number of lesions for one, the chance to get reduced RNFLT grows by 7 %.
In the logistic regression model we have put also the rest of the signs, which by experience or logic could influence the reduced RNFLT, such as EDSS, age, visual acuity, colour vision and visual field changes as well as clinical signs of optic neuritis in history; however, these signs failed to provide a statistically significant model.
Discussion
For MS patients visual disturbances and changes in the retinal nerve fibre layer are often observed, which relatively recently have been offered as biological markers for monitoring disease process. Debate continues whether this examination is required for each MS patient.
It should be noted that not always OCT method is available and hardware purchase possible. Our results show that the reduced RNFLT prediction is possible by employing routine methods of vision investigation. There is not a big surprise in the fact that the reduced RNFLT is best to foresee by using altered VEP results. VEP examination was originally referred to as an additional criterion for PPMS diagnosis (McDonald, Compston et al., 2001; Polman, Reingold et al., 2005); however, in McDonald criteria revised in 2010 was not repeatedly included (Polman, Reingold et al., 2011) and VEP role in the diagnosis of MS is reduced. From experimental autoimmune ON model, it is known that VEP amplitude reduction indicates the axonal tissue lesion, but latency prolongation is an early sign of demyelination (You, Klistorner et al., 2011).
Similarly to previous studies (Trip, Schlottmann et al., 2005; Fisher, Jacobs et al., 2006; Klistorner, Arvind et al., 2008; Naismith, Tutlam et al., 2009; Talman, Bisker et al., 2010; You, Klistorner et al., 2011), our results confirm that both VEP and OCT measurements can provide additional information on the integrity of visual ways. In literature sources there is a correlation between RNFL and the average N75 / P100 amplitude, as well as the average P100 latency both in eyes after ON and ON unaffected eyes (Naismith, Tutlam et al., 2009; Di Maggio, Santangelo et al., 2014). However, some authors approve isolated association of RNFLT with P100 latency (Fatehi, Shaygannejad et al., 2012), while others with VEP amplitude (Trip, Schlottmann et al., 2005). In studies, RNFLT has been established as a parameter with a relatively lower sensitivity compared to VEP changes. In publications describing the number of cases detected, VEP method has revealed abnormalities more frequently if compared to OCT (Naismith, Tutlam et al., 2009; Di Maggio, Santangelo et al., 2014). One of the possible explanations for the low sensitivity of RNFL is the fact that this test concerns only the beginning portion of the afferent visual ways, whereas the VEP indicates whole integrity of the visual system from the retina to the visual cortex. Therefore, if the circumstances allow to apply only one method, VEP examination is preferable; however, a combination of both methods increases the chance to diagnose the optic nerve damage. In addition, on the basis of the model developed in our study, it is possible to predict reduced RNFLT by using VEP parameters.
Although in previous studies colour vision disorders (Villoslada, Cuneo et al., 2012) and changes in visual fields (Costello, Hodge et al., 2008) statistically significantly correlated with RNFL, in our hands these figures fell outside the developed prediction models. Similarly, there are different studies on the EDSS step correlation with reduced RNFL (Grazioli, Zivadinov et al., 2008; Toledo, Sepulcre et al., 2008; Tatrai, Simo et al., 2012); however, our data show that using the EDSS step in the prediction of atrophic retinal changes is impossible.
Studies carried out confirmed the impact on the disease duration on RNFL thickness and estimated continuous decrease for about 3.7 mcm/year after ON episode (Costello, Coupland et al., 2006). However, there are also published studies claiming that the RNFL thickness is not affected by the duration of the illness (Serbecic, Aboul-Enein et al., 2010). According to our results, the chances to get reduced RNFLT increases by 5% with each year of the disease.
By analysing the MRI results, unfortunately, we took into account the total number of lesions in the brain, but did not precisely localise lesions in visual pathways. Interestingly, the atrophic changes in the MRI examination of the optic nerves did not fit into reduced RNFLT prediction model, but it should be noted that the optic nerve atrophy was determined approximately, without a precise optic nerve crosssectional area measurements.
At present, the role of OCT in monitoring of MS progress is not clear; however, by employing the proposed regression models it is feasible to provide a selective sending of patients to tertiary visual investigation centres, where OCT device is available.
Conclusions
The best choice to predict a reduced RNFLT is by using reduced N75 / P100 amplitude, prolonged P100 latency and the number of demyelinating lesions in MRI examination. Our model may be applied for identification of individuals who are at high risk of reduced RNFLT and sending them to perform OCT to tertiary MS centres. Future studies are, however, required for further validation of its practical and diagnostic utility, as well as analysis of a greater number of patients and a more precise MRI diagnostics that would improve the prediction quality of the model.
References
- Costello F. The Afferent Visual Pathway: designing a structural-functional paradigm of multiple sclerosis. ISRN Neurology, 2013: 17.
- Costello F. S., Coupland W., Hodge G. R., et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol, 2006; 59 (6): 963–969.
- Costello F. W., Hodge Y. I., Pan E., et al. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler, 2008; 14 (7): 893–905.
- Di Maggio G. R., Santangelo S., Guerrieri M., et al. Optical coherence tomography and visual evoked potentials: which is more sensitive in multiple sclerosis? Mult Scler, 2014.
- Fatehi F. V., Shaygannejad L., Mehr K., Dehghani A. Optical coherence tomography versus visual evoked potential in multiple sclerosis patients. Iran J Neurol, 2012; 11 (1): 12–15.
- Fisher J. B., Jacobs D. A., Markowitz C. E., et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology, 2006; 113 (2): 324–332.
- Grazioli E., Zivadinov R., Weinstock-Guttman B., et al. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J Neurol Sci, 2008; 268 (1–2): 12–17.
- You Y., Klistorner A., Thie J., Graham S. L. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest Ophthalmol Vis Sci, 2011; 52 (9): 6911–6918.
- Jenkins T. M. and Toosy A. T. Optical coherence tomography should be part of the routine monitoring of patients with multiple sclerosis. Mult Scler, 2014; 20 (10): 1299–1301.
- Klistorner A., Arvind H., Nguyen T., et al. Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol, 2008; 64 (3): 325–331.
- McDonald W., Compston A., Edan G., et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol, 2001; 50: 121–127.
- Naismith R. T., Tutlam N. T., Xu J., et al. Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology, 2009; 73 (1): 46–52.
- Odom J. V., Bach M., Brigell M. et al. ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol, 2010; 120 (1): 111–119.
- Polman C. H., Reingold S. C., Banwell B., et al. Diagnostic criteria for multiple sclerosis 2010: revisions to the McDonald criteria. Annals of Neurology, 2011; 69 (2): 292–302.
- Polman C. H., Reingold S. C., Edan G., et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol, 2005; 58 (6): 840–846.
- Saidha S. and Calabresi P. A. Optical coherence tomography should be part of the routine monitoring of patients with multiple sclerosis: Yes. Mult Scler, 2014; 20 (10): 1296–1298.
- Serbecic N., Aboul-Enein F., Beutelspacher S. C., et al. Heterogeneous pattern of retinal nerve fiber layer in multiple sclerosis. High resolution optical coherence tomography: potential and limitations. PLoS One, 2010; 5 (11): e13877.
- Talman L. S., Bisker E. R., Sackel D. J. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol, 2010; 67 (6): 749–760.
- Tatrai E., Simo M., Iljicsov A. J., et al. In vivo evaluation of retinal neurodegeneration in patients with multiple sclerosis. PLoS One, 2012; 7 (1): e30922.
- Toledo J., Sepulcre J., Salinas-Alaman A. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler, 2008; 14 (7): 906–912.
- Trip S. A., Schlottmann P. G., Jones S. J., et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol, 2005; 58 (3): 383–391.
- Villoslada P., Cuneo A., Gelfand J., et al. Color vision is strongly associated with retinal thinning in multiple sclerosis. Mult Scler, 2012; 18 (7): 991–999.