Human Parvovirus B19 Infection Status in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM) are chronic diseases with unclear aetiology. Human parvovirus B19 (B19) is immunomodulatory single-stranded DNA virus, which belongs to Parvoviridae family, Parvovirinae subfamily and Erythrovirus genus. B19 is considered as a possible pathogen or trigger factor in development of ME/CFS and FM.
The aim of this study was to compare frequency of B19 specific antibodies and to estimate B19 infection status in patients with ME/CFS and FM.
Thirty six patients with ME/CFS and 22 patients with FM were analysed for the presence of B19 specific IgM and IgG class antibodies in blood plasma. B19 genomic sequence was detected by nested polymerase chain reaction (nPCR). In addition, 60 apparently healthy individuals were analysed by nPCR.
B19 genomic sequence was found in 13.9 % (5/36) of patients with ME/CFS, 27.3 % (6/22) of patients with FM and only in 6.7 % (4/60) of apparently healthy individuals.
B19 specific IgG class antibodies had 63.9 % (23/36) of patients with ME/CFS and 81.8 % (18/22) of patients with FM; however, B19 specific IgG and IgM class antibodies had one patient with ME/CFS.
Assessing B19 specific IgG and IgM class antibody reaction patterns for ME/CFS patients with B19 viremia, infection status after infection was revealed in one, whereas past infection in three cases, from which two had developed NS1 antibodies, that indicates persistent B19 infection. One ME/CFS patient with B19 viremia had had infection long ago. Both presented NS1 antibodies.
One patient with FM and B19 viremia was revealed status after B19 infection, four had past infection and one – infection long ago.
In patients with ME/CFS and FM more frequently B19 infection statuses after infection (months) and past infection (months to years) were found allowing to suggest the possible involvement of the viral infection in the development of mentioned diseases.
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disease incorporating central nervous system and immune system disorders, cell energy metabolism and ion transport dysfunction, as well as cardiovascular abnormalities [Carruthers et al., 2011]. ME/CFS is characterised by severe chronic fatigue, accompanied by such clinical symptoms as sore throat, tender cervical or axillary lymph nodes, muscle pain, joint pain without swelling or redness, impaired memory or concentration, headache of new type, un-refreshing sleep, post-exertional malaise lasting more than 24 hours [Fukuda et al., 1994].
ME/CFS definition was first mentioned in 1988. After six years, this definition was revised and later other definitions were constituted. Eventually International Consensus Panel developed criteria suggesting using also term “myalgic encephalomyelitis”, due to widespread inflammation and multisystemic neuropathology of the disease [Carruthers et al., 2011].
Data on prevalence of ME/CFS varies depending on the used diagnostic criteria. Using Fukuda criteria and Reeves empirical criteria ME/CFS is determined from 0.24 % up to 2.54 % of population [Fukuda et al., 1994; Reeves et al., 2005].
Fibromyalgia (FM) is a syndrome that has a varied and inconsistent clinical spectrum. FM symptoms are described as chronic widespread pain at multiple tender points, joint stiffness and systemic symptoms such as mood swings, fatigue, cognitive dysfunction and insomnia without other explanatory diagnosis [Bellato et al., 2012].
Although knowledge and understanding about the syndrome has increased, the aetiology and pathogenesis is still unclear and diagnosing is complicated. Due to these conditions, it is believed that FM is not diagnosed for three out of four people who are suffering from this syndrome [Clauw, Arnold, McCarber, 2011]. Published reports have shown that FM affects 2–5 % of population in developed countries, furthermore majority of them are young to middle-aged women [Guymer, 2013]. Ratio between women and men who are diagnosed with this disorder is 9 : 1 [Staud, 2011]. The diagnosis is based on symptoms and existence of this medical condition has been questioned protractedly, since the causal factor of FM is still unclear and there are no standardised tests and biological markers for confirming it [Bellato et al., 2012].
Only in 1990, American College of Rheumatology (ACR) developed widely used diagnostic criteria for diagnosing FM that includes: 1) a history of widespread musculoskeletal pain present for at least three months, 2) tenderness in at least 11 of 18 defined tender points. Both criteria must be confirmed [Wolfe et al., 1990].
It is believed that important role in this disorders association with infectious agents might play cytokines and glia cells that express receptors for bacteria and viruses [Bellato et al., 2012]. Infectious agent that has been associated with ME/CFS and FM are hepatitis C virus, human immunodeficiency virus, Coxsackie B, Epstein-Barr virus, human herpesvirus 6, human parvovirus B19 (B19) and bacteria such as Borrelia, Mycoplasma and Chlamydia. However, association of single specific infectious agent and mentioned diseases has not been established [Nicolson, 2002; Ablin et al., 2006; Bansal et al., 2012; Bellato et al., 2012; Chapenko et al., 2006; Chapenko et al., 2012].
B19 is immunomodulatory single-stranded DNA virus, which belongs to Parvoviridae family, Parvovirinae subfamily and Erythrovirus genus. B19 was first discovered in 1975 in healthy donors’ blood serum [Cossart et al., 1975]. B19 genome consists of linear single-stranded DNA, 5596 bases in length. Right side of virus genome is coding viral capsid proteins VP1 and VP2, furthermore VP2 protein constitutes 95 % of the virion [Ozawa et al., 1987; Deiss et al., 1990]. Left side of B19 genome encodes non-structural proteins – NS1 that takes part in the production of infectious virus by regulating transcription and participating in replication and formation of virions DNA capsid [Momoeda et al., 1994]. After entering the cell, B19 migrates to the nucleus, where mRNA transcription and DNA replication accomplishes [Richman et al., 2002]. DNA molecules of positive and negative polarity are encapsidated and virions are released during cell lytic cycle [Green et al., 2000]. Virus replicates mainly in primary target cells – erythroid progenitor cells in the bone marrow that are permissive to B19 infection [Morey et al., 1993]. Antigenic determinant within P blood group – globoside is expressed not only on erythroblasts, but also on megakaryocytes, heart tissue, liver, lungs, kidneys, endothelium, aorta and gastro-intestinal smooth muscle tissues and synovium [Brown et al., 1993; Soderlung-Venermo et al., 2002].
B19 was first related with human disease in 1981 [Pattison et al., 1981]. It was only parvovirus associated with human diseases until 2005, when Allander with colleagues in Sweden discovered new human parvovirus called human bocavirus [Allander et al., 2005]. B19 is frequently detected in children and young people, therefore 60 to 80 % of adults has antibodies against B19 [Brown et al., 1993; Cooling et al., 1995]. B19 can cause such symptoms as rashes (exanthema subitum), infectious erythema, arthralgia, aplastic crisis with reduced red blood cell lifespan and aplasia in immunocompromised patients, various skin lesions, neutropenia, hepatobiliary diseases and neurologic diseases [Kerr, 2000].
Production of virus-specific antibodies represents protection against B19. Human normal immunoglobulin can eliminate virus from peripheral blood, therefore improving clinical signs in immunosuppressed individual [Kurtzman et al., 1989; Schwarz et al., 1990].
After primary infection, B19 can remain in organism and it has been associated with different clinical manifestations, including arthritis, fatigue and autoimmune processes [Campadelli-Fiume et al., 1999; Clark, 2000; Kerr, Tyrrell, 2003].
B19 has been considered as one of possible trigger factors for ME/CFS [Shmuel et al., 2007].
Aim
The aim of this study was to compare frequency of B19 specific antibodies and to estimate B19 infection status, as well as detect frequency of B19 genomic sequence by nPCR in patients with myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia.
Material and methods
Study was done according to safety standards and with Rīga Stradiņš University Ethics Committee’s permit issued on 27.09.2012. All enrolled patients gave their informed consent prior to the study.
Fifty eight patients: 36 patients (24/36 (67 %) female and 12/36 (33 %) male) with clinically diagnosed ME/CFS corresponding to 1994 Fukuda Centers for Disease Control and Prevention criteria and 22 patients (21/22 (95 %) female and 1/22 (5 %) male) with FM diagnosed according to 1990 American College of Rheumatology criteria were enrolled in this study.
Presence of B19 specific IgM and IgG class antibodies were detected in blood plasma by recomLine Parvovirus B19 IgM and IgG commercially available kits (MIKROGEN DIAGNOSTIK). Specific antibodies against six antigens of B19 (Vp-2p- main capsid antigen (conformation epitope); VP-N-N-terminal half of the structure proteins VP-1 and VP-2; VP-1S-specific segment (differentiation to VP-2); VP-2r- main capsid antigen (linear epitope); VP-C- C-terminal half of the structure proteins VP-1 and VP-2; NS-1- non-structure protein) were identified thereby various reaction patterns allow determining status of B19 infection.
DNA was extracted from whole blood and from cell-free blood plasma by phenol-chloroform method. Quantity of extracted DNA was measured spectrophotometrically using Nanodrop spectrophotometer. To assure the quality of DNA from whole blood and to exclude possible contamination of plasma by cellular DNA, PCR was carried out. B19 genomic sequence was detected by nested PCR (nPCR) [Hokynar et al., 2000].
In addition, 60 age and gender matched apparently healthy individuals were analysed by nPCR.
Statistical analysis was done by Fisher’s exact test.
Results
B19 genomic sequence was detected in 13.9 % (5/36) of patients with ME/CFS: three in DNA isolated from whole blood, one in DNA from cell-free blood plasma and one in both DNA form whole blood and from cell-free blood plasma; 27.3 % (6/22) of patients with FM (four in DNA isolated from whole blood and two in DNA from cell-free blood plasma. Only 6.7 % (4/60) of apparently healthy individuals had B19 genomic sequence: two in DNA isolated from whole blood and two in DNA from cell-free blood plasma (Figure 1).
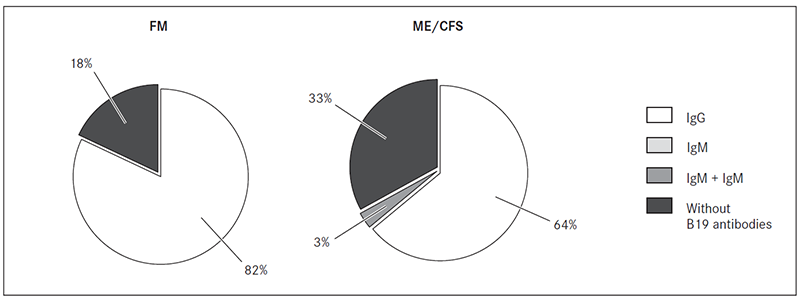
B19 specific IgG class antibodies were detected in 63.9 % (23/36) of patients with ME/CFS and 81.8 % (18/22) of patients with FM blood plasma samples. However, B19 specific IgG and IgM class antibodies had only one patient with ME/CFS.
B19 specific IgG class antibodies against NS1 protein were detected in 12/36 (33.3 %) patients with ME/CFS and 2/22 (9.1 %) patients with FM (Figure 2).
Assessing B19 specific IgG and IgM class antibody reaction patterns for patients with ME/CFS and FM with B19 genomic sequence found in DNA from whole blood and/or blood plasma, such B19 infection status as acute infection was found in none of patients with ME/CFS and FM. For one patient with ME/CFS and B19 genomic sequence detected in DNA from whole blood, infection status “after infection” (weeks to months) was revealed. B19 infection status “after infection” (months) had 11/36 (30.6 %) patients with ME/CFS and 5/22 (22.7 %) patients with FM, from them in one patient with FM B19 genomic sequence was detected in DNA isolated from whole blood.
Figure 1. Presence of B19 genomic sequence in DNA extracted from whole blood (WB) and cell-free blood plasma from patients with FM and ME/CFS and apparently healthy individuals
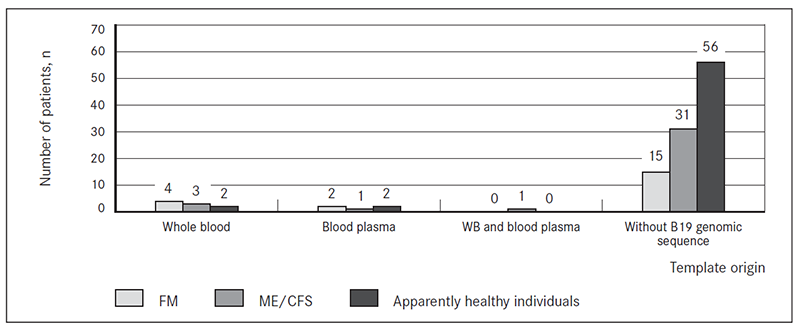
Figure 2. Frequency of B19 specific IgM and IgG class antibodies in blood plasma from patients with FM and ME/CFS
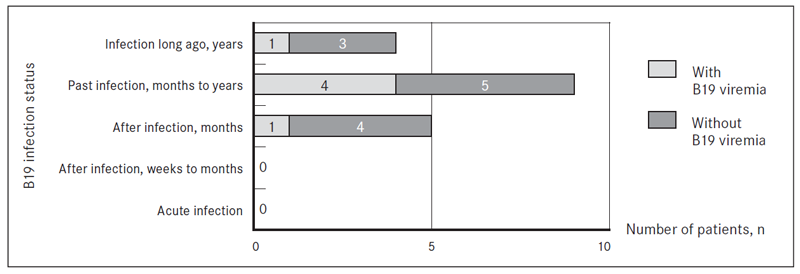
Past infection (months to years) was estimated in 8/36 (22.2 %) patients with ME/CFS and 9/22 (40.9 %) cases of FM. B19 genomic sequence was present in DNA from whole blood in 2/8 ME/CFS cases, from which one was with developed NS1 antibodies, that indicates persistent B19 infection. Another 1/8 ME/CFS patients with past infection (months to years), B19 genomic sequence was detected in DNA from cell-free blood plasma and B19 NS1 antibodies were presented. B19 genomic sequence was found also in 4/9 FM patients with past infection (months to years): 2 in DNA from whole blood and 2 in DNA from cell free blood plasma.
Four out of 36 (11.1 %) patients with ME/CFS and 4/22 (18.2 %) patients with FM had B19 infection status “infection long ago” (years). From them one patient with ME/CFS was with B19 genomic sequence in DNA from whole blood and blood plasma with developed NS1 antibodies. In addition, one patient with FM and B19 infection long ago (years) had B19 genomic sequence in DNA from whole blood (Figure 3 and Figure 4).
Figure 3. B19 infection status in viremic cases of patients with ME/CFS according to the presence of IgM and/or IgG class antibodies
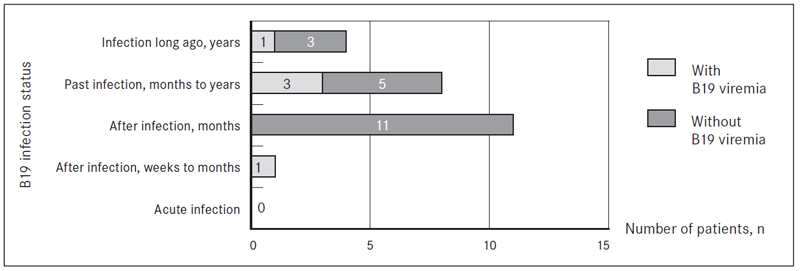
Figure 4. B19 infection status in viremic cases of patients with FM according to the presence of IgM and/or IgG class antibodies
Discussion
ME/CFS is a multi-factorial disease accompanied by severe chronic fatigue without pathophysiological explanation that reduces or subtracts ability to work. There is no consensus on presence, form and level of immune dysfunction in case of ME/CFS [Bansal et al., 2012]. ME/CFS is often followed by prolonged stress or virus infection that is considered as one of possible ME/CFS causal agents due to the fact, that most of patients report on sudden start of the illness with “flu-like” symptoms. Some virus infection can result in post-infectious fatigue and many patients with ME/CFS have immunological disturbances that could result from virus infection or be promoted by the infection. Still involvement of virus infection in aetiopathogenesis of ME/CFS remains ambiguous [Morinet, Corruble, 2012].
FM is a syndrome involving chronic, debilitating disorders that affect individuals’ quality of life, reducing ability to work, which further results in an influence on national economic situation. Various factors such as dysfunction of central and autonomic nervous system, neurotransmitters, hormones, immune system, external stressors, psychiatric and other aspects are considered to be involved in the pathogenesis of FM syndrome [Bellato et al., 2012]. Scientists consider that possible cause of fibromyalgia can be currently unknown infectious agent such as virus [Ablin, Shoenfeld, Buskila, 2006]. Using internet survey, data revealed that for 26.7 % out of 2596 of respondents with FM onset of the disease is associated with acute illness, which can possibly be caused by virus reactivation [Bennett et al., 2007]. Virus infection is considered as one of the possible causes of FM because most patients report on sudden onset of the disease and symptoms that are present in patients with chronic bacterial or viral infection. Despite the continuing studies of pathogenesis and aetiology of FM, no consensus about origin of this disorder is revealed.
According to previous study in our laboratory B19 specific IgG class antibodies was identified in 73/94 (77.7 %) of apparently healthy individuals plasma samples, IgM class antibodies – in 15/94 (16 %) from them 4 (4.3 %) had only IgM and 11 (11.7 %) had both IgM and IgG class antibodies in blood plasma [Kozireva et al., 2008]. In this study, the presence of B19 specific IgG class antibodies was statistically significantly higher in patients with ME/CFS than in apparently healthy individuals (p = 0.0036). However, significant difference was not revealed in the occurrence of B19 infection status between patients with ME/CFS and FM. It should be noted that determining B19 specific IgG and IgM class antibody reaction patterns for patients with ME/CFS and FM past infection (months to years) had more patients with FM (40.9 %), than with ME/CFS (22.2 %). Whereas status after infection (months) was defined for more patients with ME/CFS (30.6 %) than with FM (22.7 %). Furthermore, patients with B19 viremia, infection statuses “after infection” (months), “past infection” (months to years) as well as “infection long ago” were found. As for most of patients with ME/CFS and FM onset of disease occurred at least six months ago, B19 infection could serve as a trigger factor.
The study results are in accordance with other researchers’ findings of B19 IgG class antibodies frequency – 66.7 % in case of ME/CFS and 81.8 % – in FM group. Other B19 seroprevalence studies find no significant difference between patients and control group. Some report that B19 seroprevalence varies from 60 % to 80 % of population [Cooling et al., 1995], but another report shows finding of B19 specific IgG class antibodies in 74 % and IgM – in only one patient with ME/CFS. Seroprevalence of B19 is typical to general population [Zhang et al., 2010]. Kerr with colleagues analysing markers for B19 infection in 200 apparently healthy individuals and 200 patients with ME/CFS corresponding to 1994 Fukuda diagnostic criteria also found no difference in B19 seroprevalence (anti-B19 VP2 IgG class antibodies detected in 75 % and 78 %, respectively). Anti-B19 VP2 IgM class antibodies are present in four patients [Kerr et al., 2010].
Data from this study show the presence of B19 specific anti-NS1 protein antibodies in 12 (33.3 %) patients with ME/CFS and 2 (9.1 %) patients with FM, indicating possible persistence of B19 infection. In case of ME/CFS results are similar to study, where Kerr found IgG class antibodies against NS1 protein more frequently in patients with ME/CFS (41.5 %) than in healthy controls (7 %) related with high expression level of CFS-associated NHLH1 and GABPA genes. Anti-B19 NS1 IgM class antibodies are detected in three patients and one control group donor. Presence of B19 NS1 antibodies indicates of chronic or severe B19 infection, thereby part of patients’ immune system cannot sufficiently control the virus [Kerr et al., 2010].
In this study, B19 genomic sequence was detected statistically significantly more in patients with FM (6/22) than in apparently healthy individuals (4/60) (p = 0.020). Whereas no difference was found in presence of B19 genomic sequence in patients with ME/CFS (5/36) comparing with apparently healthy individuals (p = 0.288), as well as between groups of patients with ME/CFS and FM (p = 0.301).
Results show that B19 is more detected in patients with FM and ME/CFS than in apparently healthy individuals. Nevertheless, patients’ groups are comparatively small; therefore, to demonstrate statistically approved difference, investigated cohorts should be enlarged. Published studies report that using real-time PCR B19 genomic sequence is detected in 11 patients with ME/CFS and in none of control group blood donors [Kerr et al., 2010]. It is outlined that B19 can cause typical clinical symptoms of ME/CFS; therefore some studies report on this virus as one of possible trigger factors for ME/CFS, which tends to coincide with our study results [Matano et al., 2003; Shmuel et al., 2007]. Others conclude that at least part of patients B19 could cause ME/CFS, because it is detected in 40 % of patients and in less than 15 % of apparently healthy individuals [Fremont et al., 2009]. Meanwhile others find no association of parvovirus B19 infection with this disease, because the presence of B19 is not revealed in all cases of ME/CFS [Sanders, Korf, 2008]. The contrary fact is that in Brazil B19 genomic sequence was not detected in 141 Brazilian children with exanthema subitum by age four [Magalhaes et al., 2011].
Although some researchers have studied and discussed the possibility of FM and ME/CFS being one single disease, it has been proven that these are two different diseases [Abbi, Natelson, 2013]. Though finding no statistical difference comparing groups of patients with FM and ME/CFS could be explained by the similarity between FM and ME/CFS.
ME/CFS and FM could be caused by various factors and some viruses or other infectious agents may contribute to a subset for these diseases, confirming hypothesis of B19 as a trigger factor for ME/CFS and FM [Kerr et al., 2010]. At least some studies show that for part of patients B19 could be one of the trigger factors for this disease [Fremont et al., 2009].
Conclusion
In patients with myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia B19 infection statuses “after infection” (months) and “past infection” (months to years) more frequently were found allowing to suggest the possible involvement of the viral infection in the development of mentioned diseases.
This study did not detect any significant difference in the frequency of the presence of B19 specific antibodies and infection status between patients with myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia; however, it is necessary to analyse a larger cohort to draw general conclusion about the possible association of different B19 infection statuses with disease aetiopathogenesis.
Acknowledgements
The study was supported by projects: RSU ZP 13/2013 “Association of fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome with beta-herpesviruses (HHV-6A, HHV-6B, HHV-7) and parvovirus B19 infection”, “Support for doctoral study programmes and research degrees RSU” (2009/0147/1DP/1.1.2.1.2/09/IPIA/VIAA/009), Taiwan-Latvia-Lithuania Cooperation Project “Establishing of the Framework to Track Molecular Epidemiology of Parvoviruses and to Correlate Sequence Variability with Different Clinical Manifestations” Nr. 6.2.-25/2013/0039 as well as National Programme in Biomedicine.
References
- Abbi B., Natelson B. H. Is chronic fatigue syndrome the same illness as fibromyalgia: Evaluating the ‘single syndrome’ hypothesis // Quarterly Journal of Medicine, 2013; 106: 3–9.
- Ablin J. N., Shoenfeld Y., Buskila D. Fibromyalgia, infection and vaccination: Two more parts in the etiological puzzle // Journal of Autoimmunity, 2006; 27 (3): 145–152.
- Allander T., Tammi M. T., Eriksson M., et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples // Proceedings of the National Academy of Sciences of the United States of America, 2005; 102 (36): 12891–12896.
- Bansal A. S., Bradley A. S., Bishop K. N., et al. Chronic fatigue syndrome, the immune system and viral infection // Brain, Behavior and Immunity, 2012; 26 (1): 24–31.
- Bellato E., Marini E., Castolfi F., et al. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis and treatment // Pain Research and Treatment, 2012; ID 426130
- Brown K. E., Anderson S. M., Young N. S. Erythrocyte P antigen: Cellular receptor for B19 parvovirus // Science, 1993; 262 (5130): 114–117.
- Campadelli-Fiume G., Mirandola P., Menotti L. Human herpesvirus 6: An emerging pathogen // Emerging Infectious Diseases, 1999; 5: 353–366.
- Carruthers B. M., Van de Sande M. I., De Meirleir K. L., et al. Myalgic encephalomyelitis: International consensus criteria // Journal of Internal Medicine, 2011; 270 (4): 327–338.
- Chapenko S., Krumina A., Kozireva S., et al. Activation of human herpesviruses 6 and 7 in patients with chronic fatigue syndrome // Journal of Clinical Virology, 2006; 37 (1): 47–51.
- Chapenko S., Krumina A., Logina I., et al. Association of active human herpesvirus-6, -7 and parvovirus B19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome // Advances in Virology, 2012; ID205085, pp. 7. doi: 10.1155/2012/205085.
- Clauw D. J., Arnold L., McCarberg B. The science of fibromyalgia // Mayo Clinic Proceedings, 2011; 86 (9): 907–911.
- Cooling L. L., Koerner T. A., Naides S. J. Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. – The Journal of Infectious Diseases, 1995; 172 (5): 1198–1205.
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera // Lancet, 1975; 1: 72–73.
- Deiss V., Tratschin J. D., Weitz M., Siegl G. Cloning of the human parvovirus B19 genome and structural analysis of its palindromic termini // Virology, 1990; 175: 247–254.
- Fremont M., Metzger K., Hulstaert J., De Meirleir K. Detection of herpesviruses and parvovirus B19 in gastric and intestinal mucosa of chronic fatigue syndrome patients // In Vivo, 2009; 23 (2): 209–213.
- Fukuda K., Straus S. E., Hickie I., et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study // Annals of Internal Medicine, 1994; 121: 953–959.
- Green S., Malkovska I., O’Sullivan M., Brown K. Rhesus and pig-tailed macaque parvoviruses: Identification of two new members of the erythrovirus genus in monkeys // Virology, 2000; 269: 105–112.
- Guymer E. Fibromyalgia // Australian Family Physician, 2013; 42 (10): 690–694.
- Hokynar K., Brunstein J., Soderlund-Venermo M., et al. Integrity and full coding sequence of B19 virus DNA persisting in human synovial tissue // The Journal of General Virology, 2000; 81 (4): 1017–1025.
- Kerr J. R., Gough J., Richards S. C., et al. Antibody to parvovirus B19 nonstructural protein is associated with chronic arthralgia in patients with chronic fatigue syndrome/myalgic encephalomyelitis // The Journal of General Virology, 2010; 91 (4): 893–897.
- Kerr J. R., Tyrrell D. A. Cytokines in parvovirus B19 infection as an aid to understanding chronic fatigue syndrome // Current Pain and Headache Reports, 2003; 7 (5): 333–341.
- Kerr J. R. Phatogenesis of human parvovirus B19 in rheumatic disease // Annals of the Rheumatic Diseases, 2000; 59: 672–683.
- Kozireva S. V., Zestkova J. V., Mikazane H. J., et al. Incidence and clinical significance of parvovirus B19 infection in patients with rheumatoid arthritis // The Journal of Rheumatology, 2008; 35 (7): 1265–1270.
- Kurtzman G. J., Friekhofen N. K., Kimball J., et al. Pure red-cell aplasia of 10 years duration due to persistent parvovirus B19 infection and its cure with immunoglobulin // The New England Journal of Medicine, 1989; 321: 519–523.
- Magalhaes Ide M., Martins R. V., Vianna R. O., et al. Detection of human herpesvirus 7 infection in young children presenting with exanthema subitum // Memorias do Instituto Oswaldo Cruz, 2011; 106: 371–373.
- Matano S., Kinoshita H., Tangawa K., et al. Acute parvovirus B19 infection mimicking chronic fatigue syndrome // Internal Medicine, 2003; 42 (9): 903–905.
- Momoeda M., Wong S., Kawase N., et al. A putative nucleoside triphosphate-binding domain in the nonstructural protein of B19 parvovirus is required for cytotoxicity // Journal of Virology, 1994; 68: 8443–8446.
- Morey A. L., Ferguson D. J., Fleming K. A. Ultrastructural features of fetal erythroid precursors infected with parvovirus B19 in vitro: Evidence of cell death by apoptosis // The Journal of Pathology, 1993; 169 (2): 213–220.
- Morinet F., Corruble E. Chronic fatigue syndrome and viral infections. An international perspective on the future of research in chronic fatigue syndrome // http://www.intechopen.com/books/an-international-perspective-on-thefuture-of-research-in-chronic-fatigue-syndrome/chronic-fatigue-syndrome-and-viral-infections (20.06.14).
- Nicolson L. Co-infections in fibromyalgia syndrome, chronic fatigue syndrome and other chronic illnesses // Fibromyalgia Frontiers, 2002; 10 (3): 5–9, 27–28.
- Ozawa K. J., Ayub J., Yu- Shu H., et al. Novel transcription map for the B19 (human) pathogenic parvovirus // Journal of Virology, 1987; 61: 2395–2406.
- Pattison J. R., Jones S. E., Hodgson J., et al. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia // Lancet, 1981; 1: 664–665.
- Reeves W. C., Wagner D., Nisenbaum R., et al. Chronic fatigue syndrome – A clinically empirical approach to its definition and study // BMC Med, 2005; 3: 19.
- Richman D. D., Whitley R. J., Hayden F. G. Clinical Virology. – 2nd ed. – Washington: ASM Press, 2002. – Pp. 463–478.
- Sanders P., Korf J. Neuroaetiology of chronic fatigue syndrome: An overview // The World Journal of Biological Psychiatry, 2008; 9 (3): 165–171.
- Schwarz T. F., Roggendorf B., Hottentrager B., et al. Immunoglobulins in the prophylaxis of parvovirus B19 infection // Journal of Infectious Diseases, 1990; 162: 12–14.
- Shmuel A., Chapman J., Shoenfeld Y. Infection and vaccination in chronic fatigue syndrome: Myth or reality? // Autoimmunity, 2007; 40: 48–53.
- Soderlung-Venermo M., Hokynar K., Nieminen J., et al. Persistence of human parvovirus B19 in human tissues // Pathologie-Biologie, 2002; 50: 307–316.
- Staud R. Peripheral pain mechanisms in chronic widespread pain // Best Practice and Research Clinical Rheumatology, 2011; 25 (2): 155–164.
- Staud R., Smitherman M. L. Peripheral and central sensitization in fibromyalgia: Pathogenetic role // Current Pain and Headache Reports, 2002; 6 (4): 259–266.
- Watkins L. R., Milligan E. D., Maier S. F. Spinal cord glia: New players in pain // Pain, 2001; 93 (3): 201–205.
- Wolfe F., Smythe H. A., Yunus M. B., et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee // Arthritis and Rheumatism, 1990; 33 (2): 160–172.
- Zhang L., Gough J., Christmas D., et al. Microbial infections in eight genomic subtypes of chronic fatigue syndrome/ myalgic encephalomyelitis // Journal of Clinical Pathology, 2010; 63 (2): 156–164.



