Optimisation of Protocol for Isolation and Propagation of Cells from Human Breast Cancer Primary Tumour Core Biopsies
Abstract
Breast malignant tumours are heterogeneous cell populations that can be characterised by immunocytochemical and molecular markers. Sometimes treatment is not effective against some populations of malignant cells. The identification of such cells could benefit treatment in future. Therefore, our aim was to elaborate protocol for isolation of various breast cancer cell populations from breast tumour core biopsy samples.
Mechanical and enzymatic disaggregation was performed and cells were seeded at various densities on collagen-coated plates.
This study presents data on cell counts, seeding efficiency, observed morphology and primary immunocytochemical results for the cells isolated from breast cancer core biopsies.
Isolated cell counts vary among biological samples obtained from different donors. Cultured cells represent mostly fibroblast-like and stromal-like cell morphology. They produce characteristic hormone receptors ER, PR and Her2 (ErbB2) and have a weak signal for cell differentiation marker CD24.
In future, the protocol will be advanced using specific media to improve isolation of epithelioid type cells.
Introduction
Breast cancer is the most common type of cancer among women in Latvia. According to current data of Disease Prevention and Control Centre of Latvia, 1133 new cases of breast cancer were registered in 2013, and in Latvia altogether there are 13,434 registered breast cancer patients (Smate et al., 2013). Breast cancer is a heterogeneous group of neoplastic cells originating from the epithelial cells that are lining breast milk ducts. There is a high degree of diversity between and within tumours, as well as among cancer patients (Polyak, 2011). Different strategies for therapy exist; however, many breast tumours are not responsive or eventually develop resistance to therapy. Therefore, it is essential to identify treatment-resistant breast cancer cells, to characterise them, and to determine differences between therapy-sensitive cells.
There is a variety of strategies for isolating primary cell cultures that depend on cellular properties, e.g., adhesion ability, differences in density, and the antibody binding capacity (Tomlinson et al., 2012). Another common method with a high efficiency rate (~ 50 %) for primary culture establishment is explant cultures. Explant cultures is a technique that has several significant advantages, for example, it maintains cellular migration and preserves the histotypic relationships among various cell types within an organ of origin without changing the tissue architecture, which is caused by enzymes (Pei et al., 2004).
Human breast cancer-derived cells is a widely used experimental tool that enable to gain specific, clinically important information. Attempts to establish breast cancer cell lines from primary tumours have always been a challenging task. Poor efficiency is often due to various factors: (1) the extraction of viable tumour cells from surrounding stroma; (2) slow proliferation rate of breast cancer in vivo which is maintained after transferring of cells into culture. Due to slow proliferation rate of primary breast cancer cells, it is essential to prevent overgrowth of normal stromal cells (e.g., fibroblasts) that can overtake breast cancer cells, therefore selective media is a prerequisite; (3) the different hormone and growth factor requirements of breast cancer cells from different patients and different breast cancer types and subtypes; (4) the importance of factors secreted by normal cells that negatively influence proliferation of breast cancer cells. These and other factors make primary cultures of breast cancer cells in vitro a much more difficult task than the culturing normal mammary epithelial cells (Ethier et al., 2000).
For successful cell propagation it is essential to adjust culture conditions that can vary in-between cell lines. Therefore, suitable cell culture media and supplements are of utmost importance. It is also important to note that most cells are substrate-dependent, and substrate is an important factor for optimal culture conditions (Liberio et al., 2014). To mimic in vivo environment and to promote cell adhesion, collagen coated surfaces can be used (Chandrasekaran et al., 1999). Within tissues, cells interact with each other and with the extracellular matrix. For maintenance of normal cell interaction and signalling, the key factor is seeding density as it may affect cell extracellular production, their metabolism and reduce viability (Issa et al., 2011).
This study presents data of pilot experiments to isolate primary cells from the breast cancer core biopsy samples. Different seeding density on collagen coating of the culturing plates was tested.
Aim
The aim of the study was to develop protocol for isolation and propagation of various cell populations from breast cancer core biopsy tissue samples.
Material and Methods
Reference cell cultures. Established human breast cancer cell lines MDA-MB-231 and MCF7 were used as reference cell lines for tumour cells (morphological and immunocytochemical characterisation). Dermal fibroblasts (a kind gift from Cell Transplantation centre, PSKUS) were used as fibroblast control.
Cell isolation from core biopsies. Breast cancer core biopsies were obtained from Pauls Stradins Clinical University Hospital, Centre of Breast Diseases. The study was approved by the Ethics Committee of the hospital. From each patient, who signed informed consent form, there were two core biopsy samples obtained: transversal (central) and tangential (peripheral) in relation to tumour node centre. Tissues were minced and enzymatically disaggregated using collagenase solutions. Type III collagenase (1.0–1.5 mg/ml) was used for enzymatic digestion of tissues from tumour peripheral samples. Type II collagenase/dispase (1 mg/ml/20 U/ml) was used for the central biopsy sample. Enzymatic treatment was performed overnight at room temperature and followed by incubation at 37°C until complete disaggregation of the sample. Samples were filtered and seeded on collagen-coated plates (6-well and 96-well) in DMEM:F12 (1 : 1) containing 10 % fetal bovine serum (FBS), 1 % 1x penicillin/streptomycin (10,000 U/ml), 0.002 mM L-glutamine and 2.5 mg/l Amphotericin B solution at 37 °C, 5 % CO2.
Development of explant cultures. One half of the tissues from peripheral sample of tumour was used to initiate explant cultures. Tissues were cut into small (approx. 1 mm2) pieces that were placed on 24-well plate (1 explant/well). Fetal bovine serum was applied on the explant and incubated for 1–2 h at 37°C, 5 % CO2. DMEM:F12 (1 : 1) containing 10 % FBS was added to the explants and they were incubated overnight at 37 °C, 5 % CO2. After observation under microscope, the unattached explants were attached to the surface of the well using sterile scalpel. Media change was performed every 2–3 days.
Immunocytochemistry. Protein localisation was detected using AbCam Mouse and rabbit specific HRP detection IHC kit (Cat. No: ab93677). In brief, cells were seeded on 96-well plate with seeding density of 500–1000 cells/well. After reaching 70–80 % confluence, they were fixed using ice-cold ethanol. None of the antibodies used in this research required antigen retrieval. Cells were washed, blocked with Protein block. Primary antibody was added (we used 1 : 200 dilution for all antibodies) and incubated for 1 h at the room temperature. Biotinylated secondary antibody was added and incubated for 10 min at room temperature. Cells were stained with DAB substrate kit (ab64238) and hematoxylin.
Statistical analysis. Statistical analysis was performed using IBM SPSS Statistics, Version 21. Exploratory statistical analysis was performed for various biopsy sample disaggregation protocols.
Results
Disaggregation of breast cancer core biopsy samples
During the study period, central and peripheral core biopsy samples from nine patients were received in the laboratory.
Central core biopsy samples from all nine patients were disaggregated enzymatically with collagenase II and dispase combination. Peripheral core biopsy samples from four patients were disaggregated enzymatically with collagenase III. Only half of the sample was used for enzymatic disaggregation (collagenase III for one sample, and collagenase II/dispase for four samples) for five peripheral core biopsy samples, the other half of the biopsy sample was used for initiation of primary explant cultures. The samples were exposed to enzyme solution until complete or almost complete disaggregation of sample and reaching relatively homogenous cell suspension, as determined visually.
The mean number of cells obtained from the biopsy samples after enzymatic disaggregation was highly variable: 2.33 ± 2.78, × 105 (range: 0.02–9.20, × 105) cells per biopsy, resulting in coefficient of variation of 119 %. The numbers of cells obtained as a result of enzymatic disaggregation of biopsy samples for various isolation protocols are shown in Table 1.
Table 1. Disaggregation of breast cancer core biopsy samples: enzymes used for disaggregation, length of disaggregation, and isolated cell counts
| Protocol | Biopsy samples | ||
|---|---|---|---|
| n | Cell count, × 105 cells (Mean ± SEM | Range | |
| Enzymes | |||
| Collagenase II + dispase | 13 | 2.10 ± 0.68 | 0.02–8.80 |
| Collagenase III | 5 | 2.95 ± 1.68 | 0.06–9.20 |
| Length of enzymatic disaggregation | |||
| ≤ 12 h | 6 | 3.72 ± 1.68 | 0.50–9.20 |
| > 12 h | 12 | 1.64 ± 0.48 | 0.02–5.56 |
Enzymes for disaggregation of biopsy samples. Collagenase II in combination with dispase was used for disaggregation of 13 biopsy samples (9 central and 4 peripheral); collagenase III was used for disaggregation of five peripheral biopsy samples.
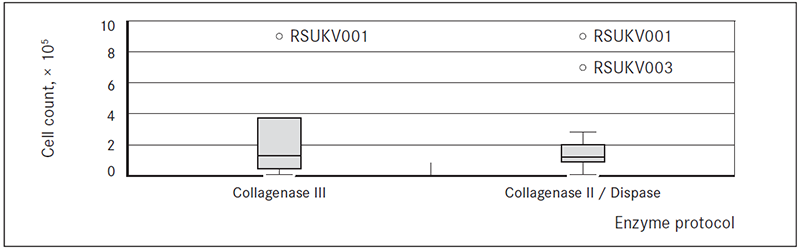
The average numbers of cells obtained after disaggregation with collagenase III and collagenase II / dispase did not differ significantly and were, respectively, 2.95 ± 0.61, × 105 (range: 0.06– 9.20, × 105) and 2.10 ± 0.68, × 105 (range: 0.02–8.80, × 105) cells per biopsy sample (adjusting for material used for explant cultures) (Figure 1).
Figure 1. Boxplots of cell counts after tissue disaggregation with two different enzyme sets: collagenase III, and collagenase II/dispase
Length of enzymatic disaggregation. On average it took 2.33 ± 0.66 hours (range 2.50–43.80 hours) to completely or almost completely disaggregate a sample.
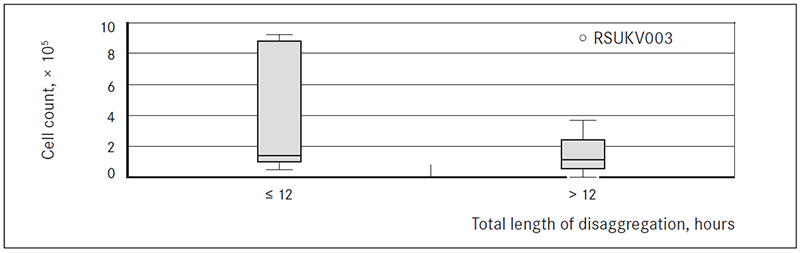
For biopsies exposed to enzymatic disaggregation for ≤ 12 hours and for > 12 hours the average numbers of cells obtained per biopsy were respectively 3.72 ± 1.7, × 105 (range: 0.50–9.20, × 105) and 1.64 ± 0.48, × 105 (range: 0.02–5.56, × 105) cells (Figure 2).
Figure 2. Boxplot of cell counts after different length of disaggregation: ≤ 12 hours or > 12 hours
Cell counts for transversal and tangential biopsy samples did not differ significantly
The average number of cells obtained from the transversal and tangential biopsies after enzymatic disaggregation varied widely, and did not differ significantly.
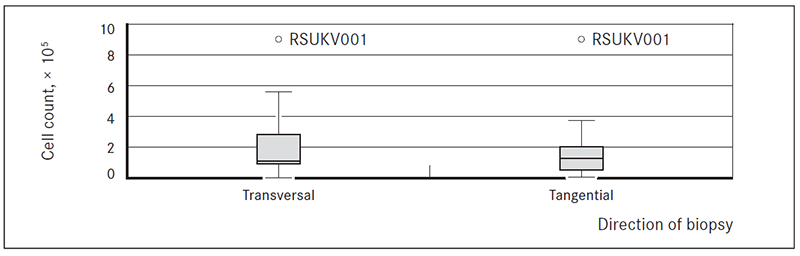
The average number of cells (± standard error of mean) per biopsy (adjusting for material use for explant cultures) was 2.48 ± 0.96 × 105 (95 % CI: 0.25 × 105, 4.70 × 105) for transversal biopsies (range: 0.02–8.80, × 105) and 2.2 ± 0.95 × 105 (95 % CI: 0.01 × 105, 4.37 × 105) for tangential biopsies (range: 0.06–9.20, × 105) (Figure 3).
Figure 3. Boxplots of cell counts from tissues of transversal and tangential breast cancer core biopsies
Primary culture of cell populations after enzymatic disaggregation
After disaggregation of biopsy samples, cells were seeded on 96-well and 6-well plates. Seeding density on 96-well plates varied among biopsies. To observe clonogenic capacity of the cells, we seeded cells on 96-well plates with different densities: from 2 to 1900 cells/well, i.e., between 7–6550 cells cm2, respectively. Cloning efficacy for cell seeding in densities from 2 to 60 cells per well (n = 10) in general was very low – cell attachment was observed for 20–30 % of wells, but cells were nonviable and/or nonproliferating. Therefore, efficacy of clonogenic cell cultures very early in cell line isolation process in our experiments, using DMEM: F12 with 10 % FBS and 1 % glutamine as growth medium and collagen coating as adherent substrate, was close to 0 %.
The results for cell seeding efficacy at higher cell densities – 1800 and 1900 cells per well, i.e., 6200 and 6550 cells/cm2 – for representative transversal and tangential, respectively, biopsy samples are shown in Table 2.
Cell seeding efficacy was characterised by cell attachment on day 10 (number and proportion of wells with attached cells) and cell growth on day 30–35 (number and proportion of wells with various levels of cell confluence).
Table 2. Efficacy of cell seeding on 96-well plates in high density (1800–1900 cells/well) for representative transversal and tangential core biopsy sample cell populations
| Characteristics of cell seeding efficacy | Transversal biopsy, number of wells | Tangential biopsy, number of wells | ||
|---|---|---|---|---|
| N = 480 | % | N = 480 | % | |
| Cell attachment on day 10 | 228 | 47.50 | 25 | 5.21 |
| Cell confluence on day 30–35, including: | 232 | 48.33 | 24 | 5.00 |
| < 1 % – nonproliferating cells | 80 | 16.67 | 6 | 1.25 |
| 1–20 % – very low growth rate | 64 | 13.33 | 2 | 0.42 |
| 21–40 % – slow growth rate | 26 | 5.42 | 8 | 1.67 |
| 41–60 % – moderate growth rate | 19 | 3.96 | 2 | 0.42 |
| 61–80 % – high growth rate | 17 | 3.54 | 4 | 0.83 |
| > 81 % – very fast growth rate | 30 | 6.25 | 2 | 0.42 |
The recovery rates after seeding on 6well plates at the moment of publication were established for only some, but not all biopsies. Cells seeded in high or very high densities on 6-well plates gave 7.08 % to 300 % cell yield, demonstrating highly variable growth rate of different cell populations from different patients.
Primary culture of cell populations from tissue explants
To explore if enzymatic disaggregation has any impact on breast core biopsy cell population viability and growth, we performed also experiments to obtain primary cultures from tissue explants. For initiation of explant cultures, we used half of sample from five tangential breast cancer core biopsies. The efficacy of explant cultures as judged by cell population outgrowth from explant is shown in Table 3.
Efficacy of explant cultures differed among different patients, and was between 0.0–66.7 %. For the explant cultures with near-confluent or confluent cell outgrowth (n = 3), we performed subcultivation, including cell count, as shown in Table 3.
Table 3. Explant cultures of breast cancer biopsy samples
| Biopsy No. | Number of explants | Explants with cell outgrowth on day 30 | Cell yield, × 105 cells | |
|---|---|---|---|---|
| n | % | |||
| RSUKV005M | 12 | 9 | 66.7 | 0.60 |
| RSUKV006M | 12 | 1 | 8.3 | < 0.01 |
| RSUKV007M | 6 | 0 | 0.0 | 0.00 |
| RSUKV008M | 6 | 2 | 33.3 | ND* |
| RSUKV009M | 6 | 4 | 66.7 | ND* |
* ND – not determined: at the time of publication confluence of cell outgrowth is too low to perform subculture.
Isolated cells exhibited variable morphology
Cells were cultured on collagen-coated surfaces. Fibroblast-like phenotype was the most abundant among all samples (Figure 4A). Also in several cases sphere-forming-like cell aggregates (squamospheres) were observed (Figure 4B). During cultivation of isolated cells from one biopsy tissue material variable cell morphology was detected (Figure 4C and 4D). In some cases separate, dense cell colonies were observed (Figure 4 E). Rarely, epithelial-like cells were observed (Figure 4F).
Reference breast cancer cell line MCF7 has a cobblestone-like phenotype with a pronounced strong cell-cell adhesion (Figure 4G). Cells of another reference cell line MDA-MB-231 phenotypically appear as elongated spindle-shaped cells much a like to fibroblasts with a pronounced cellular scattering (Figure 4H). Fibroblasts appear as large in size, spindle-shaped cells (Figure 4I).
Cell outgrowth from the tissue sample explants was observed from the first week in culture. Most cells exhibited fibroblast-like morphology (Figure 5). Epithelial (or cobblestone) phenotype, characteristic for cancer cells was observed.
Immunocytochemical analysis of ER, PR, HER2 and CD24
Cells of reference cultures MCF7 and MDA-MB-231, dermal fibroblasts and central and peripheral biopsy tissues were stained for typical markers of breast cancer (ER, PR, HER2) and for CD24 (Figure 6).
Oestrogen receptor (ER) staining was most abundant in the reference culture MCF7. Very weak staining was observed for another reference cell line of triple-negative breast cancer MDA-MB-231, whereas moderate ER staining was detected in both breast cancer cells, as well as in dermal fibroblasts (Figure 6, row 2, F–J).
Another typical marker of breast cancer cells – progesterone receptor (PR) was detected in all samples. Weaker staining was observed for reference cell line MDA-MB-231 (Figure 6, row 3, K–O).
Very strong staining of HER2 (ErbB2) was observed for MCF7 cells, whereas strong staining – for dermal fibroblasts, and weak staining was observed for cells isolated from representative biopsy and MDA-MB-231 cells (Figure 6, row 4, P–U).
Staining with cell differentiation marker CD24 gave very weak signal in all cell samples tested (Figure 6, row 5, V–Z).
Figure 4. Representative morphologies of cells isolated and cultured from breast cancer core biopsy (A–F) and reference culture cells (G–I).* G – MCF7 cells, H – MDA-MB-231 cells, I – dermal fibroblasts
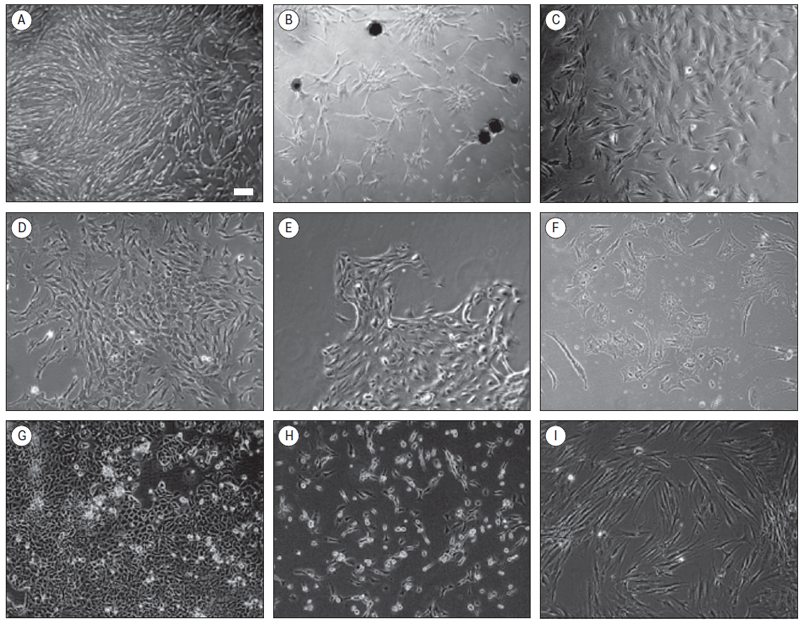
* Scale bar in section A, 100 μm
Figure 5. Representative image of cell outgrowth from the explant on day 7 after seeding.*
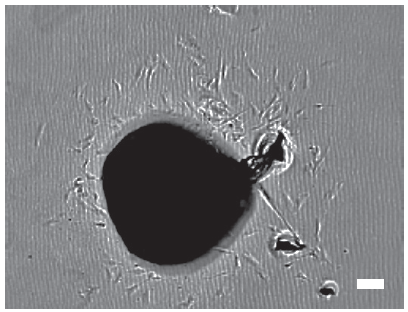
* Scale bar 100 μm
Figure 6. Immunocytochemical analysis of breast cancer markers*
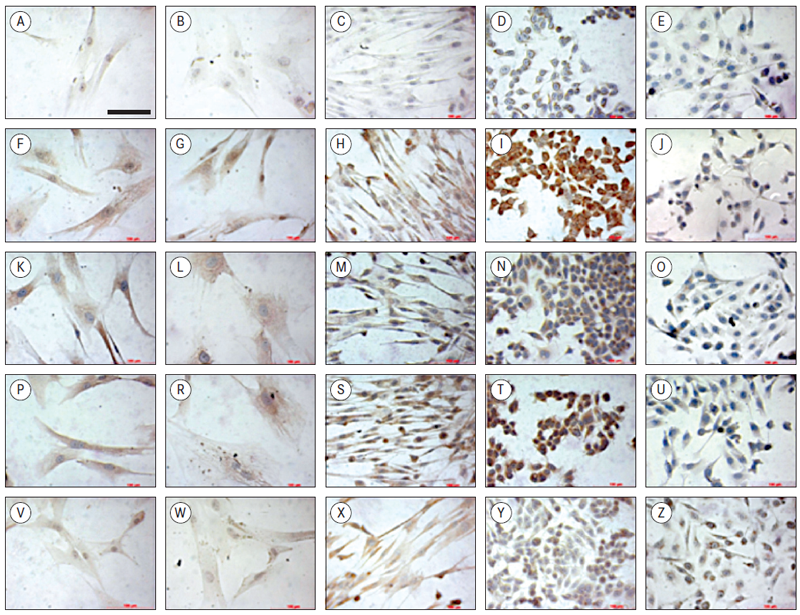
* Scale bar in section A applies to all sections, 100 μm
Horizontally: row 1 (A–E) represents all negative controls; row 2 (F–J) staining with ER; row 3 (K–O) – cells stained with PR; row 4 (P–U) cells stained with HER2 and row 5 (V–Z) – cells stained with CD24.
Vertically: column 1 (A–V) – cells from the transversal tumour biopsy cultured for 5 passages; column 2 (B–W) – cells from the tangential tumour biopsy cultured for 5 passages; column 3 (C–X) – dermal fibroblasts; column 4 (D–Y) – MCF7 cells, and column 5 (E–Z) – MDA-MB-231 cells.
Discussion
Skilfully obtained (under the ultrasound control) breast cancer core biopsies contain representative cell populations that can serve as a good cell source for further investigation of heterogeneity of the tumour. To compare cell isolation strategies in different parts of the tumour, biopsies were collected from the peripheral and from the central part of the tumour (Oakes et al., 2014).
Collagenase type III was used for disaggregation of tissue from peripheral part of tumour (tangential biopsy sample) (Liu et al., 2007). Gentler approach was taken to disaggregate tissues from the central part of the tumour (transversal biopsy). Mix of different enzymes, usually collagenase and dispase or hyaluronidase has been described previously (Goldstein et al., 2011; Stingl et al., 2005). On average, there were no significant differences in cell counts after use of the two enzyme protocols (Figure 1). However, tissues disaggregated faster when collagenase II/dispase cocktail was used. Length of enzymatic disaggregation had no significant impact on number of cells obtained from the sample, although it showed slight trend towards lower number of cells with increase of length of digestion time (Figure 2). Also there were no significant differences observed in numbers of cells depending of tissue or biopsy type (transversal vs. tangential biopsy); however, slight trend towards lower cell count in tangential biopsy samples in comparison to transversal biopsy samples was observed (Figure 3). This suggests that further development of protocol should include an enzyme cocktail consisting of collagenase and dispase or hyaluronidase, and shorter digestion times for both types of biopsies.
There are several recognised problem areas to solve to obtain efficient epithelial and cancer cell cultures. Nutrients required by cancer cells may differ from those required by equivalent normal cells, and removal of stromal elements and supportive cells may deprive tumour and epithelial cells of substrate (matrix), nutrients and growth factors necessary for their survival. Seeding of epithelial and / or tumour cells in low densities may dilute growth factors produced by adjacent similar (i.e., malignant) and supportive cells, and deprive cells of close contacts necessary for successful growth; therefore, usually closely interacting population is required (Freshney, 2010).
Similar difficulties and key factors have also been found to be specific for breast cancer cultures – cell density dependence for growth, slow proliferation rate in vivo which is maintained after transferring the cells into culture, different hormone and growth factor requirements of cells from different patients, and importance of factors secreted by normal cells (Ethier et al., 2000). These culture growth difficulties were observed also in our efforts to obtain clonogenic individual cell lines from primary cells. Even after seeding of cells in quite high densities, the attachment and growth of cells was comparatively low. Furthermore, at least 60–70 % of cell populations with high or very high growth rates morphologically were fibroblast-type or mixed fibroblast and mesenchymal-type cells (Figure 4).
Results described in this report suggest that propagation of mixed cell populations in highdensities could be appropriate for early breast cancer cell line isolation stages due to density-dependent growth and slow-growth rates. Also, further research directions will include different growth media and supplements, as DMEM:F12 and fetal bovine serum as universal media does not meet specialised nutritional and growth factor requirements of various breast cancer cell populations, and various substrates, e.g., Matrigel (extracellular matrix) and feeder layers of fibroblasts or fetal intestinal cell lines, as more complete imitation of extracellular contacts needed for epithelial and breast cancer cells.
The explant cultures showed various efficacy (Table 3), but closer study of cell populations obtained after enzymatic and mechanical disaggregation of breast cancer core biopsy samples is still in progress, so at the moment of the publication we cannot make any conclusions whether enzymatic disaggregation exerts any selection pressure on cell populations obtained from the biopsy sample and decreases their heterogeneity, i.e., representativeness of cell line library obtained from the patient. Also, to explore improvement of efficacy of explant cultures, use of various cultivation media for cell outgrowth is planned in the nearest future.
Immunocytochemistry analysis was performed to do the primary characterisation of cultured cells from representative transversal biopsies and tangential biopsies. Although this method is not quantitative, it is still possible to compare the staining if they are performed simultaneously.
Breast cancer cell lines MCF7 and MDA-MB-231 were used as positive controls. Dermal fibroblasts were stained to obtain the information about marker expression in non-epithelial cells. After morphological analysis we already could predict that most of our cells exhibit fibroblast-like or stromallike phenotype. Similarly, the expression of breast cancer markers was very close in intensity among representative isolated breast cancer cells and dermal fibroblasts. Whereas situation was different with reference cell line MCF7 that are of epithelial origin. According to literature, these cells have strong ER and PR expression (Subik et al., 2010) that is in line with our data. However, these MCF7 cells usually do not exhibit strong expression of HER2. It has been shown that elevated levels of HER2 in MCF7 can stimulate the activation of anti-apoptotic gene expression (Siddiqa et al., 2008); however, this direction was not further tested.
MDA-MB-231 is the reference cell line for triple negative type breast cancer, and the very weak hormone receptor staining is in line with overall known information (Subik et al., 2010).
CD24 is one of the cell differentiation markers that has been used in combination with CD44 as prospective cancer stem cell marker for basal/mesenchymal cell lines MCF7 and MDA-MB-231 (Al-Hajj et al., 2003; Sheridan et al., 2006). It has been shown that CD44+/CD24low/negative cancer cells exhibit enhanced invasive properties. The low expression of CD24 in MCF7 and MDA-MB-231 is in line with literature data (Siddiqa et al., 2008). CD24 is expressed by the range of cells (Fang et al., 2010) and is associated with mesenchymal stem cells (Wetzig et al., 2013). It has been previously described that dermal fibroblasts resemble several mesenchymal stem cell features, like cell plasticity by differentiation towards adipogenic, osteogenic and chondrogenic lineages (Chang and Guo, 2014). This similarity could explain CD24 expression in our dermal fibroblasts. Overall, the expression patterns of different breast cancer markers in our isolated cells were very similar to the ones observed for dermal fibroblasts. Nevertheless, morphologically they differ. More elaborate tests are needed to draw any conclusions about the origins of isolated cells.
Conclusions
Development of patient-specific representative breast cancer cell cultures from core biopsies is challenging mainly due to differences among patients, and the small volume of tissue sample (therefore small cell numbers). Shorter enzymatic disaggregation time, higher seeding density, surface features and cell type specific media could be more effective and will be tested in future experiments.
Acknowledgments
The present work is supported by the RSU project “Reconstruction of breast cancer genetic evolution based on proteomics in cancer cell cultures”.
References
- Al-Hajj M., Wicha M. S., Benito-Hernandez A., Morrison S. J. et al. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 2003; 100 (7): 3983–3988.
- Chandrasekaran S., Guo N. H., Rodrigues R. G., Kaiser J., et al. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin-like growth factor-1 and CD98. J Biol Chem, 1999; 274 (16): 11408–11416.
- Chang Y., Guo Z. Mesenchymal stem cell-like properties in fibroblasts. Cell Physiol Biochem, 2014; 34 (3):703–714.
- Ethier S. P., Ammerman Ch. A., Dziubinski M. L. Isolation and culture of human breast cancer cells from primary tumors and metastases. Methods in Mammary Gland Biology and Breast Cancer Research / Ed. by Ip M. M. and Asch B. B. New York: Plenum Publisher, 2000, 195–208.
- Fang X., Zheng P., Tang J., Liu Y. CD24: from A to Z. Cellular and Mol Immunol, 2010; 7 (2): 100–103.
- Freshney I. Culture of tumor cells. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. 6th ed. New Jersey: John Wilwy & Sons, Inc., 2010, 463–479.
- Goldstein A. S., Drake J. M., Burnes D. L., Finley D. S., et al. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nat Protoc, 2011; 6 (5): 656–667.
- Issa R. I., Engebretson B., Rustom L., McFetridge P. S., et al. The effect of cell seeding density on the cellular and mechanical properties of a mechanostimulated tissue-engineered tendon. Tissue Eng, Part A, 2011; 17 (11–12): 1479–1487.
- Liberio M. S., Sadowski M. C., Soekmadji C., Davis R. A., et al. Differential effects of tissue culture coating substrates on prostate cancer cell adherence, morphology and behaviour. PLoS One, 2014; 9 (11): e112122.
- Liu R., Wang X., Chen G., Dalerba P., et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med, 2007; 356 (3): 217–226.
- Oakes S. R., Gallego-Ortega D., Ormandy C. J. The mammary cellular hierarchy and breast cancer. Cell. Mol Life Sci, 2014; 71 (22): 4301–4324.
- Pei X. F., Noble M. S., Davoli M. A., Rosfjord E., et al. Explant-cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In Vitro Cell Dev Biol Anim, 2004; 40 (1–2): 14–21.
- Polyak K. Heterogeneity in breast cancer. J Clin Invest, 2011; 121 (10): 3786–3788.
- Sheridan C., Kishimoto H., Fuchs R. K., Mehrotra S. CD44+/CD24− breast cancer cells exhibit enhanced invase properties: an early step necessary for metastasis. Breast Cancer Research, 2006; 8 (5): R59.
- Siddiqa A., Long L., Li L., Marciniak R. A., et al. Expression of HER-2 in MCF-7 breast cancer cells modulates antiapoptotic proteins Survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signalling pathways. BMC Cancer, 2008; 8: 129.
- Stingal J., Emerman J. T., Eaves C. J. Enzymatic dissociation and culture of normal human mammary tissue to detect progenitor activity. Methods Mol Biol / Ed. by Helgason C. D., Miller C. L. 3rd ed. Totowa: Human Press Inc., 2005, 249–263.
- Subik K., Lee J. F., Baxter L., Strzepek T., et al. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl), 2010; 4: 35–41.
- Smate I., Mozgis Dz. u. c. Onkoloģija. Statistikas dati par pacientu skaitu sadalījumā pa reģioniem, lokalizācijas veidiem, dzimuma un vecuma grupām no 2009. gada līdz 2013. gadam (eng. Oncology. Statistical data about patients’ regional distribution, types of localisation, gender and age between 2009 and 2013). http://www.spkc.gov.lv/veselibasaprupes-statistika/. (accessed on 20.05.2015.)
- Tomlinson M. J., Tomlinson S., Yang X. B. and Kirkham J. Cell separation: terminology and practical considerations. J Tissue Eng, 2012; 4: 2041731412472690.
- Wetzig A., Alaiya A., Al-Alwan M., Pradez C. B., et al. Differential marker expression by cultures rich in mesenchymal stem cells. BMC Cell Biol, 2013; 14: 54.



