Digestive System Diseases as the most Common Disease Group among Patients with Extended-spectrum Beta-lactamase Producing Bacterial Infection
Digestive System Diseases as the most Common Disease Group among Patients with Extended-spectrum Beta-lactamase Producing Bacterial Infection
Abstract
Extended spectrum beta-lactamase (ESBL) producing bacteria are associated with increased antimicrobial resistance.
The aim of this study was to characterise ESBL-producing bacterial infection cases by analysing all consecutive ESBL-producing bacteria isolation cases during 6-month period, using patient medical records and bacteriological material.
136 isolation cases were obtained from 110 hospitalisation episodes and 108 different patients. ESBL-producing Enterobacteriaceae were isolated from 52 (38.2 %) female and 84 (61.8 %) male patients with the mean age of 61.35 ± 16.92 years. ESBL-producing Klebsiella pneumoniae (48.5 %) was mostly isolated from wound biomaterial (32.4 %). Digestive system diseases were the most commonly found disease group, accounting for 49.26 % cases. Patients spent an average of 56.80 ± 67.19 days at hospital, in 82 cases (60.29 %) patients were admitted to the Intensive Care Unit (ICU) and spent there 24.35 ± 30.69 days. In 28 cases (20.6 %) patients died as a hospitalisation outcome at the age of 70.43 ± 13.23 years. Higher risk for worse hospitalisation course and outcome in patients with ESBL-producing bacterial infection was observed in patients younger than 65 years, patients with injuries, musculoskeletal and infectious diseases.
The study allows for following conclusions:
- In Latvia, the prevalence of Kl. pneumoniae is higher comparing to other European countries.
- Longer hospital stay, more frequent admission to the ICU and longer ICU stay is associated with higher mortality in patient population with ESBL-producing bacterial infection.
- Digestive system diseases are the most common disease group among patient population with ESBL-producing bacterial infection.
- Risk factors associated with worse hospitalisation course and outcome in patient population with ESBL-producing bacterial infection are: patient age under 65 years, injuries, musculoskeletal and infectious diseases.
Introduction
Since the first outbreak in 1983 (Germany), extended-spectrum beta-lactamase (ESBL) producing Gram negative bacteria (Enterobacteriaceae) reports have increased due to the increasing consumption of antimicrobials and widespread gene mutation, providing bacterial resistance to extended spectrum penicillins and cephalosporins (Coque et al., 2008; Canton et al., 2008).
ESBL are mostly produced by Enterobacteriaceae, mainly by Escherichia coli and Klebsiella pneumoniae (Canton et al., 2008; Shaikh et al., 2015; Lee et al., 2012; Spanu et al., 2002). ESBL-producing bacteria predominate in cases of urinary tract infections and ventilator-associated pneumonia, but these bacteria can cause also a wide variety of other nosocomial and community acquired infections (Osthoff et al., 2015; Spadafino et al., 2014).
Risk factors for ESBL-producing bacterial infection acquisition include severe underlying diseases, immunosuppression, prior administration of antibiotics, long stay in hospital, nursing home, intensive care unit, presence of catheters and longer stay in the intensive care unit (Spadafino et al., 2014; Skippen et al., 2006; Tacconelli et al., 2014).
Antibacterial resistance differs from region to region and according to ECDC Surveillance 2013 report data more than 50 % of Kl. pneumoniae and 10–25 % of E. coli strains in Latvia produce ESBL and are resistant to third generation cephalosporins (Coque et al., 2008; Canton et al., 2008; Spanu et al., 2002; Weist et al., 2012).
Bacterial phenotypes and genotypes, which determine ESBL-producing bacteria prevalence and antimicrobial sensitivity vary widely depending on geographic location, hospital, ward, patient group or even type of infection (Canton et al., 2008; Shaikh et al., 2015; Lee et al., 2012; Hawkey et al., 2009).
Only a few studies concerning ESBL-producing bacterial infection molecular epidemiology have been conducted in the Baltic region (Lillo et al., 2014; Paberza et al., 2007). Most of them include Pauls Stradins Clinical University Hospital and cover the period before 2012, where the number of ESBLproducing bacteria isolation cases at Rīga Eastern Clinical University hospital (RECUH), the largest hospital in Latvia, is growing and have not been studied before.
Aim
The aim of the study was to obtain general characterisation (demographic, epidemiological, bacteriological, disease, hospitalisation course and outcome background) of patients with ESBL-producing bacterial infection and specify risk factors for worse hospitalisation course and outcome in ESBLproducing bacterial infection cases in RECUH – the biggest hospital in Latvia.
Material and Methods
A cross-sectional single centre study was conducted at Rīga Eastern Clinical University Hospital (RECUH), including all consecutive ESBL-producing bacteria isolation cases collected from Bacteriology laboratory over a 6-month period, dating from September 1, 2013 till March 1, 2014.
All patient cases regardless of patient age, gender and clinical severity with ESBL-producing bacteria found in the tested biomaterial were included in the study. All patient cases where ESBL-producing bacteria were not found in the biomaterial were excluded from the study. The number of ESBL-producing bacteria isolation cases included in the study accounted for 43% of all ESBL-producing bacteria cases isolated during one-year period in RECUH.
Data sources included bacteriological biomaterial (wound biomaterial, urine, bronchoalveolar fluid, abdominal cavity biomaterial, blood, abscess, cerebrospinal fluid and sputum) for ESBL-producing Enterobacteriaceae strain identification and patient medical records for demographic, hospitalisation and disease data collection. Data were collected in a database using MS Excel 2013 software, based on an originally designed study protocol and questionnaire, containing 106 parameter groups, including demographic, epidemiological, clinical, disease and bacteriological parameter groups. Statistical analysis was performed with SPSS version 20.0, using Spearman’s correlation coefficient, Mann–Whitney U test and Pearson’s Chi-square test.
Bacteriological analysis was performed according to the EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, version 1.0, December 2013 and provided information about the biomaterial that was tested, as well as ESBLproducing Enterobacteriaceae strains identified during the study.
Information about the diseases was gathered from official patient discharge documentation and grouped according to ICD-10 (International Statistical Classification of Diseases) version 2015 (ICD-10, 2015). Each disease was encoded separately and summed up in total disease count for statistical analysis.
Hospitalisation course in patients was evaluated according to the length of the hospital stay, ICU stay and ICU admission. Hospitalisation outcome in patients was evaluated according to mortality.
The study was reviewed and accepted by the Rīga Eastern Clinical University Hospital Ethics committee.
Results
Epidemiological and demographic data
A total of 136 ESBL-producing bacteria cases were isolated from 110 hospitalisation episodes and 108 different patients. 98 ESBL-producing bacteria cases (72.1 %) were isolated from Clinic “Gaiļezers”, 17 cases (12.5 %) – from Oncology Centre of Latvia and 21 cases (15.4 %) – from Clinic “Bikernieki”.
ESBL-producing Enterobacteriaceae in 52 (38.2 %) cases were isolated from female and 84 (61.8 %) cases – male patients with the mean age of 61.35 ± 16.92, ranging from 22 to 89 years.
Bacteriological testing data
ESBL-producing bacteria was mostly isolated from wound biomaterial (n = 44; 32.35 %) (Figure 1).
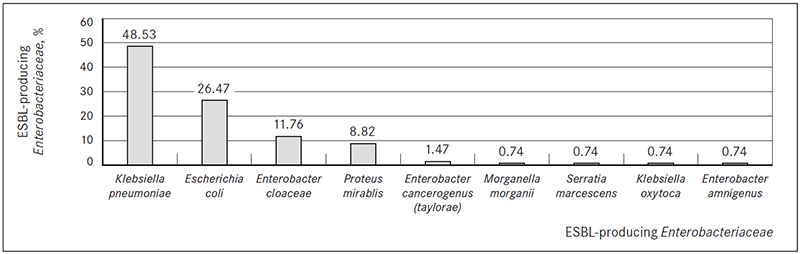
In most cases ESBL-producing Kl. pneumoniae (n = 66, 48.53 %) and E. coli (n = 36; 26.47 %) were isolated (Figure 2).
Figure 1. Biomaterial for ESBL-producing Enterobacteriaceae isolation
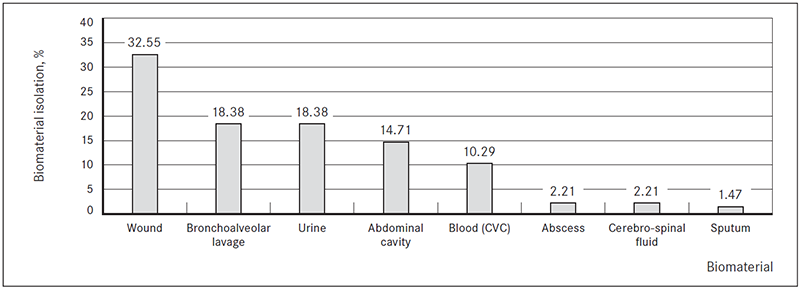
Figure 2. ESBL-producing Enterobacteriaceae isolated
Hospitalisation data
Patients spent an average of 56.80 ± 67.19 days at the hospital, ranging from 1 to 390 days during each hospitalisation period. In 82 ESBL-producing bacteria isolation cases patients (60.29 %) were admitted to the ICU at least 1 time, ranging from 1 to 3 times each hospitalisation period and spent there an average of 24.35 ± 30.69 days, ranging from 1 to 141 days each hospitalisation period. Most of them (n = 64, 78.05 %) were admitted to the ICU one time and 54 patients (39.71 %) were never admitted to the ICU during their hospitalisation period.
In 28 ESBL-producing bacteria isolation cases (20.6 %) patients died as a hospitalisation outcome at the mean age of 70.43 ± 13.23, ranging from 46 to 96 years.
Diseases
Patients in most ESBL-producing bacteria isolation cases had digestive system diseases (n = 67, 49.26 %), respiratory system diseases (n = 64, 47.06 %) and diseases of the circulatory system (n = 63, 46.36 %) (Figure 3).
Figure 3. Disease groups (ICD-10) found in patients with ESBL-producing bacterial infection, %
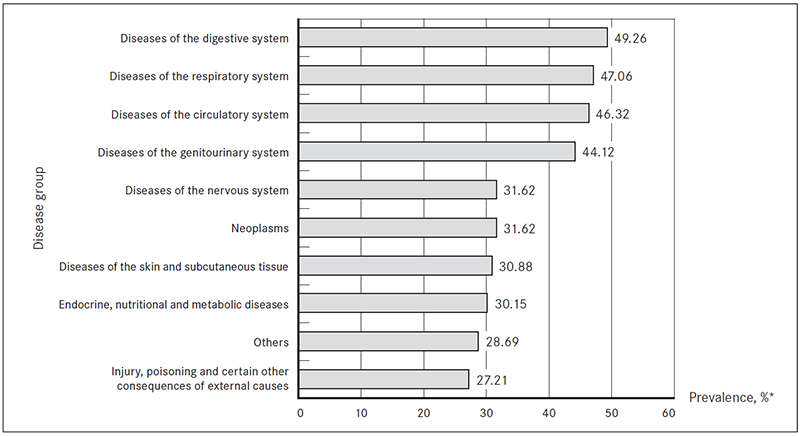
* Prevalence of each disease group was analysed within all ESBL-producing bacteria isolation cases.
The most common digestive system diseases (n = 101) observed in patient population with ESBLproducing bacterial infection were disorders of gallbladder, biliary tract, pancreas and spleen, diseases of small and large intestine and diseases of the peritoneum. Most common group of digestive diseases were chronic (n = 11; 10.9 %) and acute (n = 15; 14.9 %) pancreatitis with complications, including sub-diaphragmatic (n = 6; 5.9 %) and retroperitoneal (n = 4; 4 %) abscesses, acute necrotic collections (n = 3; 3 %) and pancreatic fistula (n = 1; 1 %). Frequently found disorders also included mechanical ileus (n = 11; 10.9 %) with complications, including transverse colon perforation (n = 5; 5 %), ileum perforation (n = 1; 1 %) and intestinal necrosis (n = 1; 1 %); acute cholecystitis (n = 5; 5 %) and choledocholithiasis (n = 9; 8.9 %) with complications, including mechanical icterus (n = 7; 6.9 %) and cholangitis (n = 3; 3 %); as well as intra-abdominal abscesses (n = 5; 5 %). Other gastrointestinal diseases included ulcerative colitis, primary sclerosing cholangitis, diverticulosis, haemoperitoneum and their complications (n = 14; 14 %).
In 29 ESBL-producing bacteria isolation cases (21.32 %) patients had another multi-drug resistant (MDR) microorganism co-infection. In 16 cases (55.17 %) patients were co-infected with multi-drug resistant Acinetobacter baumannii (MRAB), in 10 cases (34.48 %) – methicillin-resistant Staphylococcus aureus (MRSA) and in 3 cases (10.34 %) Clostridium difficile infection (Figure 4).
Figure 4. Multi-drug resistant (MDR) microorganism co-infections in patients with ESBL-producing bacterial infection, %
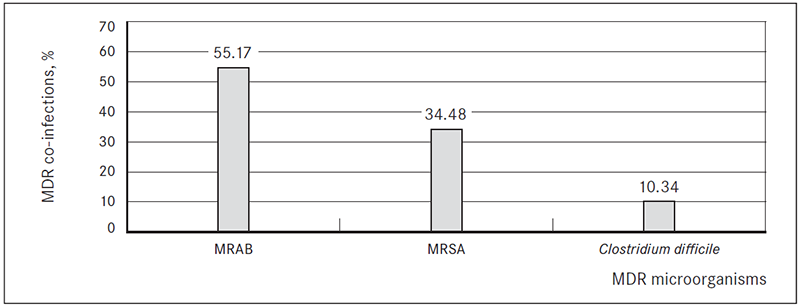
In 60 cases (44.12 %), patients had at least one surgery within the studied hospitalisation period, ranging from one to four surgeries each hospitalisation period. In 60 cases (44.12 %) patients had had at least one surgery in the past (before the studied hospitalisation period), ranging from one to six surgeries each hospitalisation period.
In 32 ESBL-producing bacteria isolation cases (23.53 %) patients had sepsis, in 23 cases (16.91 %) – MODS (multi organ dysfunction syndrome) and in 18 cases (13.24 %) – shock, mostly septic and traumatic shock.
Disease association with epidemiologic data
Female patients had more diseases in general, including ESBL-producing bacterial infection and concomitant diseases, more circulatory system diseases, neurologic diseases and more endocrine diseases, including diabetes, where male patients had more neoplasms and more mental and behavioural disorders (Table 1).
Younger patients suffered more from mental and behavioural disorders, injuries and shock. Older patients had more neoplasms, circulatory system diseases, neurologic diseases, genitourinary system diseases, MDR infections and diabetes (Table 2).
More diseases at the same time, especially circulatory system diseases, respiratory system diseases, musculoskeletal system diseases and neurologic diseases, as well as shock, MODS and sepsis were associated with higher mortality. Whereas people with circulatory diseases, genitourinary system diseases and more diseases at the same time, including ESBL-producing bacterial infection cases and concomitant diseases died at an older age (Table 3).
Table 1. Comparison of the most common underlying conditions in female and male patients with ESBL-producing Enterobacteriaceae infection
| Female patients | p value |
|---|---|
| More diseases | p = 0.017 |
| Circulatory system diseases | p = 0.055 |
| Neurologic diseases | p = 0.045 |
| Endocrine diseases | p < 0.001 |
| Diabetes | p = 0.006 |
| Male patients | p value |
|---|---|
| Neoplasms | p = 0.013 |
| Mental and behavioural disorders | p = 0.033 |
Table 2. Comparison of the most common underlying conditions in the patient age groups with ESBL-producing Enterobacteriaceae infection
| Patients younger than 65 years | p value |
|---|---|
| Mental and behavioural disorders | p = 0.026 |
| Injuries | p = 0.001 |
| Shock | p = 0.003 |
| Patients older than 65 years | p value |
|---|---|
| Neoplasms | p < 0.001 |
| Circulatory system diseases | p < 0.001 |
| Neurologic diseases | p = 0.009 |
| Genitourinary system diseases | p < 0.001 |
| MDR infections | p = 0.003 |
| Diabetes | p = 0.014 |
Table 3. Medical conditions associated with mortality in patients with ESBL-producing Enterobacteriaceae infection
| Conditions associated with increased mortality | p value |
|---|---|
| More diseases | p = 0.002 |
| Circulatory system diseases | p < 0.001 |
| Respiratory system diseases | p = 0.006 |
| Musculoskeletal system diseases | p = 0.002 |
| Neurologic diseases | p = 0.003 |
| Shock | p = 0.039 |
| MODS | p < 0.001 |
| Sepsis | p = 0.001 |
| Conditions associated with later age of death | p value |
|---|---|
| More diseases | p = 0.018 |
| Circulatory system diseases | p = 0.003 |
| Genitourinary system diseases | p = 0.002 |
The most frequently found circulatory system diseases included arterial hypertension, coronary heart disease, myocardial infarction, atrial fibrillation, chronic heart disease, intra-cerebral haemorrhage, cerebral aneurysms, atherosclerosis and aneurysms of peripheral arteries. The most frequently found neurologic diseases included Parkinsonism, Parkinson’s disease, vascular dementia, polyneuropathy, myelitis, cerebral abscesses, acute and chronic cerebral ischemia, encephalitis, meningitis and epilepsy. The most frequently found endocrine diseases, included type 1 and type 2 diabetes, Cushing’s disease, adrenal insufficiency, colloidal goitre, autoimmune thyroiditis, hypothyroidism and adiposity. The most frequently found neoplasms included colorectal cancer, laryngeal cancer, breast cancer, prostate adenocarcinoma, anaplastic ependymoma, Hodgkin’s lymphoma and uterine cancer. The most frequently found mental and behavioural disorders included delirium, psychosis and psychoorganic syndrome. The most frequently found injuries included multiple bone fractures, burns, cerebral contusions and commotions, subdural hematomas, epidural hematomas, subarachnoid haemorrhages, ruptured spleen and liver. The most frequently found genitourinary system diseases included chronic glomerulonephritis, pyelonephritis, cystitis, acute renal failure and nephrolithiasis. The most frequently found musculoskeletal system diseases included osteoarthritis, psoriatic polyarthritis, gout, spondylosis, spondyloarthritis, spinal stenosis, ankylosing spondyloarthritis, scoliosis, osteoporosis and osteomyelitis.
Disease association with hospitalisation course and outcome
Patients with more respiratory system diseases, infectious and parasitic diseases, injuries, poisoning and certain other consequences of external causes and additional MDR infection spent more days at hospital. In addition, patients with more surgeries and MODS spent more days at hospital. Patients with musculoskeletal system and connective tissue diseases and patients with genitourinary system diseases spent fewer days at hospital (Table 4).
Patients with more diseases were admitted to the ICU more frequently, including patients with digestive system diseases, infectious and parasitic diseases, sepsis, MODS and shock. Patients with diabetes were admitted to the ICU less frequently (Table 5).
Patients with multiple diseases spent more days in the ICU, including patients with respiratory system diseases, MDR infections, mental and behavioural disorders, sepsis, MODS, shock, as well as patients who underwent more surgeries during their hospital stay. Patients with diabetes, musculoskeletal system and connective tissue diseases, neoplasms and genitourinary system diseases spent less days in the ICU (Table 6).
Patients with injury, poisoning and certain other consequences of external causes were admitted to the ICU fewer times (p = 0.022), but spend more days there (p < 0.001).
Table 4. Medical conditions associated with the length of hospitalisation in patients with ESBL-producing Enterobacteriaceae infection
| Longer hospitalisation period | p value |
|---|---|
| Respiratory system diseases | p = 0.001 |
| Infectious and parasitic diseases | p = 0.047 |
| Injuries | p < 0.001 |
| Additional MDR infection | p < 0.001 |
| More surgeries | p = 0.012 |
| MODS | p < 0.025 |
| Shorter hospitalisation period | p value |
|---|---|
| Musculoskeletal system diseases | p = 0.029 |
| Genitourinary system diseases | p = 0.038 |
Table 5. Medical conditions associated with ICU admission in patients with ESBL-producing Enterobacteriaceae infection
| More frequent admission to the ICU | p value |
|---|---|
| More diseases | p = 0.014 |
| Digestive system diseases | p = 0.002 |
| Infectious diseases | p = 0.047 |
| Sepsis | p < 0.001 |
| MODS | p = 0.006 |
| Shock | p = 0.003 |
| Less frequent admission to the ICU | p value |
|---|---|
| Diabetes | p = 0.027 |
Table 6. Medical conditions associated with the length of the ICU stay in patients with ESBL-producing Enterobacteriaceae infection
| Longer ICU stay | p value |
|---|---|
| More diseases | p = 0.001 |
| Respiratory system diseases | p < 0.001 |
| MDR infection | p < 0.001 |
| Mental and behavioural disorders | p = 0.048 |
| MODS | p < 0.001 |
| Shock | p < 0.001 |
| More surgeries | p = 0.025 |
| Shorter ICU stay | p value |
|---|---|
| Diabetes | p = 0.027 |
| Musculoskeletal system diseases | p = 0.004 |
| Neoplasms | p = 0.003 |
| Genitourinary system diseases | p = 0.008 |
Discussion
Before analysing certain result categories in detail, it is important to note the common principles that could explain the results obtained in the study. The biomaterial in most of ESBL-producing bacteria isolation cases was gathered from surgical profile departments, where most of the diseases accounted for digestive system disease group (ICD-10). This, together with the fact that 15% of the biomaterial was gathered from the wound clinic, may have resulted in higher wound biomaterial prevalence for ESBL-producing bacteria isolation. The results could also be directly associated with common medical practice in Latvia – to obtain biomaterial and perform bacteriological testing mostly from surgical sites and wounds, opposed to other biomaterial – urine, blood and bronchoalveolar fluid, which is mostly bacteriologically tested in cases of serious infection or unfavourable course of treatment. Therefore, some of ESBL-producing bacteria isolation cases from wound biomaterial could be associated with ESBLproducing bacteria colonisation. In these cases hospitalisation course and outcome, including length of hospital stay, ICU stay, ICU admission and even mortality, could not be associated with ESBL-producing bacterial infection, but more with the main disease, determining the clinical severity.
On the other hand, many studies in literature also analyse just the consecutive ESBL-producing bacteria isolation cases and associate differences with geographical diversity of ESBL-producing bacteria. In addition, the fact that patient age and gender in this study is similar to patient populations with ESBLproducing bacterial infection researched in other studies allows us to speculate about geographical differences in ESBL-producing bacteria phenotype. Although we try to analyse and find geographical differences among ESBL-producing bacteria, we should always keep in mind that these geographical differences might also be explained by local common medical practice, which determines the indications for biomaterial collection and bacteriological testing.
Epidemiological, demographic and bacteriological testing data
Mean age and gender in the studied patient cohort with ESBL-producing bacterial infection corresponded to the data found in other literature sources. ESBL-producing Enterobacteriaceae were mostly isolated from male patients around 60 years of age (Skippen et al., 2006).
Kl. pneumoniae was the most frequently isolated ESBL-producing microorganism in this study, where E. coli was the most frequently isolated ESBL-producing strain in other studies (Moor et al., 2008; Sader et al., 2014).
This difference could be explained by different biomaterials and medical conditions researched in various studies. Wound biomaterial was the most frequently studied biomaterial in this study, where urine was the most common biomaterial for ESBL-producing bacteria isolation in other studies (Ruiz de Alegria et al., 2011; Kassakian et al., 2014).
Hospitalisation course and outcome data
Patients in this study stayed at hospital more than 2 times longer (Ruiz de Alegria et al., 2011), were admitted to the ICU at least 3 times more frequently (Mehrgan et al., 2008) and stayed in the ICU more than 2 times longer (Mehrgan et al., 2008) than in other ESBL-producing bacterial infection studies. In addition, mortality rates were higher than found in literature (Ruiz de Alegria et al., 2011).
These differences could be explained by the different periods taken into account when analysing the days spent at hospital. In this study all hospitalisation period from the day of admission until the day of discharge was taken into account, where in several other studies – only days after the ESBL-producing bacteria isolation was determined. The differences could also be explained by the fact that in many studies biomaterial was gathered only from the ICU, where in this study biomaterial was gathered from the whole hospital – including ICU, as well as regular therapy and surgery wards. The differences could also be explained by the variety of biomaterials used for ESBL-producing bacteria isolation and medical conditions associated with ESBL-producing bacterial infection. The authors of this study acknowledge in some cases ESBL-producing bacterial infection was not always the main condition that determined the clinical severity and outcome for a patient, but acted as a serious risk factor for less favourable hospitalisation course and outcome due to a more challenging empirical antimicrobial therapy.
The mean age of death in patient population with ESBL-producing bacterial infection has never been described in literature before. As mentioned before, in this study the mean age of death was determined for both ESBL-producing bacterial infection and possible colonisation cases; therefore, in some cases other underlying disease may have influenced patients’ age of death.
Disease data
The most frequently found disease group in patient cohort with ESBL-producing bacterial infection in this study was digestive system diseases. Some literature sources state biliary tract diseases (Ruiz de Alegria et al., 2011; Kassakian et al., 2014; Peralta et al., 2012; Kolar et al., 2006), liver cirrhosis (Moor et al., 2008; Peralta et al., 2012) and intra-abdominal infection (Ruiz de Alegria et al., 2011; Kassakian et al., 2014; Peralta et al., 2012) as conditions associated with ESBL-producing bacterial infection, but none of them have been described as frequent. The most common disease group associated with ESBL-producing bacterial infection and colonisation in literature was genitourinary tract diseases (Moor et al., 2008; Ruiz de Alegria et al., 2011; Kassakian et al., 2014; Peralta et al., 2012; Kolar et al., 2006). Respiratory tract diseases (Moor et al., 2008; Ruiz de Alegria et al., 2011; Kassakian et al., 2014; Peralta et al., 2012; Kolar et al., 2006), cardiovascular diseases (Moor et al., 2008; Ruiz de Alegria et al., 2011; Kassakian et al., 2014; Peralta et al., 2012; Kolar et al., 2006), neoplasms, diabetes, nervous system diseases, primary bacteremia (bloodstream infection) (Ruiz de Alegria et al., 2011; Kassakian et al., 2014; Peralta et al., 2012; Kolar et al., 2006) and sepsis (Kolar et al., 2006) were other frequently found diseases and conditions associated with ESBL-producing bacterial infection found both in this and other studied in the literature (Moor et al., 2008; Peralta et al., 2012). The higher digestive system disease prevalence and lower genitourinary system disease prevalence in our study could be explained by different biomaterial ESBL-producing Enterobacteriaceae were isolated from in various studies (Ruiz de Alegria et al., 2011; Kassakian et al., 2014).
Slightly more patients in this study were co-colonised or co-infected with other MDR bacteria (Meyer et al., 2011) than found in other literature sources. The co-infection rate with Methicillin-resistant Staphylococcus aureus (MRSA) found in this study was approximately the same as found in other literature sources (Meyer et al., 2011). Co-infection cases with Pseudomonas aeruginosa and Vancomycin-resistant Enterococcus (VRE) faecium have been reported in literature, but co-infection with these pathogens was not found in this study. On the other hand, co-infection with Multi-resistant Acinetobacter baumannii (MRAB) and Clostridium difficile was found in this study, but have not been described in literature before. The higher MDR infection prevalence in this study could be explained by the difference in biomaterial ESBL-producing Enterobacteriaceae were isolated from in this study (mostly wound material) on other studies (mostly urine material) (Ruiz de Alegria et al., 2011; Kassakian et al., 2014).
The higher surgical operation prevalence in this study comparing to other literature sources (Moor et al., 2008; Mehrgan et al., 2008) could be explained by the fact that more ESBL- producing Enterobacteriaceae were isolated from surgical profile patients. The difference also could be explained by the biomaterial source for ESBL-producing bacteria isolation – more wound and abdominal cavity material was used in this study, comparing to urine and bronchoalveolar biomaterial in other studies (Ruiz de Alegria et al., 2011; Kassakian et al., 2014; Mehrgan et al., 2008).
Hospitalisation course and outcome data association with epidemiologic, demographic and disease data
Relationship stating that mental and behavioural disorders, injury, poisoning and certain other consequences of external causes, neurologic diseases and genitourinary system diseases prevail in younger (< 65 years) patients, and neoplasms, as well as circulatory system diseases and MDR infection diseases are found in older (> 65 years) patients is also observed in the general population (NSDUH, 2013; Warner et al., 2005; MacDonald, 2000; Liu et al., 2012; National Cancer Institute, 2015; Thu Trang et al., 2013; Countries et al., 2010; Imai et al., 2009) and, therefore, cannot be considered as specific characteristics for patient population with ESBL-producing bacterial infection.
During this study certain risk factors associated with prolonged hospital stay, more frequent ICU admission, longer ICU stay and higher mortality were identified.
Risk factors associated with longer hospital stay included: (1) younger age (< 65 years), (2) more time spent in the ICU, (3) respiratory system diseases, (4) infectious and parasitic diseases, (5) injuries, poisoning and certain other consequences of external causes, (6) additional MDR infections, (7) more surgeries and (8) MODS.
Risk factors associated with more frequent ICU admission included: (1) older age (> 65 years), (2) more time spent at hospital, (3) more diseases, (4) digestive system diseases, (5) infectious and parasitic diseases, (6) sepsis, (7) MODS and (8) shock.
Risk factors associated with longer ICU stay included: (1) younger age (< 65 years), (2) more diseases, (3) injury, poisoning and certain other consequences of external causes, (4) respiratory system diseases, (5) mental and behavioural disorders, (6) additional MDR infections, (7) more surgeries, (8) sepsis, (9) MODS and (10) shock.
Risk factors that were associated with higher mortality rate as hospitalisation outcome were: (1) patients admitted to the ICU more frequently, (2) patients spending more days in the ICU, (3) more diseases, (4) circulatory system diseases, (5) respiratory system diseases, (6) musculoskeletal system diseases, (7) neurologic diseases, (8) sepsis, (9) MODS and (10) shock.
Some risk factors have been studied and found in the general population and, therefore, are not specific for patient population with ESBL-producing bacterial infection. Respiratory system diseases, mental and behavioural disorders, additional MDR infections, multiple diseases, more surgeries, sepsis, MODS and shock have been mentioned as risk factors for longer hospital and ICU stay in the general population (Kwizera et al., 2012; Du et al., 2013). Older patient age, longer time at hospital, more diseases, digestive system diseases, sepsis, MODS and shock have been described as reasons for more frequent ICU admission in studies concerning general population (Kwizera et al., 2012; Du et al., 2013). More frequent ICU admission rate, longer ICU stay, more diseases, circulatory system diseases, neurologic diseases, respiratory system diseases, sepsis, MODS and shock have been described as risk factors for higher mortality in general population before (Mayr et al., 2006).
Patients younger than 65 years, injuries, musculoskeletal and infectious diseases have never been described in literature before as risk factors associated with worse hospitalisation course and outcome in patient population with ESBL-producing bacterial infection. Although this might be a novel fining, the authors of this study note that ESBL-producing bacterial infection in many cases were found in severely injured patients with musculoskeletal diseases and infectious comorbidities – mostly young male patients. Therefore, these factors should be considered as risk factors for worse hospitalisation course and outcome in patient population with ESBL-producing bacterial infection all together as a risk factor combination.
Conclusions
- In Latvia, the prevalence of ESBL-producing Kl. pneumoniae is higher comparing to other European countries.
- Longer hospital stay, more frequent admission to the ICU and longer ICU stay is associated with higher mortality in the studied patient population with ESBL-producing bacterial infection.
- Digestive system diseases are the most common disease group in the studied patient population with ESBL-producing bacterial infection.
- Risk factors associated with worse hospitalisation course and outcome in the studied patient population with ESBL-producing bacterial infection include patients younger than 65 years, injuries, musculoskeletal and infectious diseases.
References
- AYA – National Cancer Institute. http://www.cancer.gov/types/aya (12.05.2015).
- Cantón R., Novais A., Valverde A., et al. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect, 2008; 14: 144–153. doi:10.1111/j.1469-0691.2007.01850.x.
- Coque T. M., Baquero F., Canton R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill Bull Eur Sur Les Mal Transm = Eur Commun Dis Bull, 2008; 13: 1–11. doi:10.1128/AAC.49.7.2693-2700.2005.
- Countries I., Fuster V., Kelly B. B. Epidemiology of cardiovascular disease. Washington (DC): National Academies Press (US); 2010.
- Du B., An Y., Kang Y., et al. Characteristics of critically ill patients in ICUs in mainland China. Crit Care Med, 2013; 41: 84–92. doi:10.1097/CCM.0b013e31826a4082.
- Hawkey P. M., Jones A. M. The changing epidemiology of resistance. J Antimicrob Chemother, 2009; 64 (Suppl 1): i3–10. doi:10.1093/jac/dkp256.
- ICD-10 Version: 2015. http://apps.who.int/classifications/icd10/browse/2015/en (29.05.2015).
- Imai S., Ito Y., Ishida T., et al. High prevalence of multidrug-resistant Pneumococcal molecular epidemiology network clones among Streptococcus pneumoniae isolates from adult patients with community-acquired pneumonia in Japan. Clin Microbiol Infect, 2009; 15: 1039–1045. doi:10.1111/j.1469-0691.2009.02935.x.
- Kassakian S. Z., Mermel L. A. Changing epidemiology of infections due to extended spectrum beta-lactamase producing bacteria. Antimicrob Resist Infect Control, 2014; 3: 9. doi:10.1186/2047-2994-3-9.
- Kolar M., Latal T., Cermak P., et al. Prevalence of extended-spectrum beta-lactamase-positive Klebsiella pneumoniae isolates in the Czech Republic. Int J Antimicrob Agents, 2006; 28: 49–53. doi:10.1016/j.ijantimicag.2006.02.012.
- Kwizera A., Dünser M., Nakibuuka J. National intensive care unit bed capacity and ICU patient characteristics in a low income country. BMC Res Notes, 2012; 5: 475. doi:10.1186/1756-0500-5-475.
- Lee J. H., Bae I. K., Lee S. H. New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med Res Rev, 2012; 32: 216–232. doi:10.1002/med.20210.
- Lillo J., Pai K., Balode A., et al. Differences in Extended-Spectrum Beta-Lactamase Producing Escherichia coli Virulence Factor Genes in the Baltic Sea Region 2014. 2014.
- Liu Q., Li Z., Wang H., et al. High prevalence and associated risk factors for impaired renal function and urinary abnormalities in a rural adult population from southern China. PLoS One, 2012; 7: e47100. doi:10.1371/journal.pone.0047100.
- MacDonald B. K. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain, 2000; 123: 665–676. doi:10.1093/brain/123.4.665.
- Mayr V. D., Greil V., Jochberger S., et al. Causes of death and determinants of outcome in critically ill patients. Crit Care, 2006; 10: R154. doi:10.1186/cc5086.
- Mehrgan H., Rahbar M. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli in a tertiary care hospital in Tehran, Iran. Int J Antimicrob Agents, 2008; 31: 147–151. doi:10.1016/j.ijantimicag.2007.09.008.
- Meyer E., Ziegler R., Mattner F., et al. Increase of patients co-colonised or co-infected with methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium or extended-spectrum β-lactamase-producing Enterobacteriaceae. Infection, 2011; 39: 501–506. doi:10.1007/s15010-011-0154-0.
- Moor C. T., Simmons G., Briggs S., et al. Extended-spectrum beta-lactamase (ESBL)-producing enterobacteria: factors associated with infection in the community setting, Auckland, New Zealand. J Hosp Infect, 2008; 68: 355–362. doi:10.1016/j.jhin.2008.02.003.
- Osthoff M., McGuinness S. L., Wagen A. Z., Eisen D. P. Urinary tract infections due to extended-spectrum beta lactamase-producing gram-negative bacteria: identification of risk factors and outcome predictors in an Australian tertiary-referral hospital. Int J Infect Dis, 2015. doi:10.1016/j.ijid.2015.03.006.
- Paberza R., Selderi S., Leja S., Storozenko J. Prevalence of extended-spectrum β-lactamase producing Enterobacteriaceae strains in Latvia. Bioautomation, 2007, 99–103. http://www.clbme.bas.bg/bioautomation/2007/vol_7.1/files/7_…
- Peralta G., Lamelo M., Velasco M., et al. Impact of empirical treatment in extended-spectrum beta-lactamaseproducing Escherichia coli and Klebsiella spp. bacteremia. A multicentric cohort study. BMC Infect Dis, 2012; 12: 245. doi:10.1186/1471-2334-12-245.
- Weist K., Muller A., Monnet D., et al. Surveillance of antimicrobial consumption in Europe 2012. http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1174 (20.05.2015.).
- Ruiz de Alegría C., Rodríguez-Baño J., Calvo J., et al. Klebsiella pneumoniae strains producing extendedspectrum beta-lactamases in Spain: microbiological and clinical features. J Clin Microbiol, 2011; 49: 1134–1136. doi:10.1128/JCM.02514-10.
- Sader H. S., Farrell D. J., Flamm R. K., Jones R. N. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn Microbiol Infect Dis, 2014; 78: 443–448. doi:10.1016/j.diagmicrobio.2013.11.025.
- Sasirekha B. Prevalence of ESBL, AmpC β-lactamases and MRSA among uropathogens and its antibiogram. EXCLI Journal, 2013; 12: 81–88 – ISSN 1611-2156.
- Shaikh S., Fatima J., Shakil S., et al. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci, 2015; 22: 90–101. doi:10.1016/j.sjbs.2014.08.002.
- Skippen I., Shemko M., Turton J., Palmer C., et al. Epidemiology of infections caused by extended-spectrum β-lactamaseproducing Escherichia coli and Klebsiella spp.: a nested case-control study from a tertiary hospital in London. J Hosp Infect, 2006; 64: 115–123. doi:10.1016/j.jhin.2006.05.010.
- Spadafino J. T., Cohen B., Liu J., Larson E. Temporal trends and risk factors for extended-spectrum beta-lactamaseproducing Escherichia coli in adults with catheter-associated urinary tract infections. Antimicrob Resist Infect Control, 2014; 3: 2012–2015. doi:10.1186/s13756-014-0039-y.
- Spanu T., Luzzaro F., Perilli M., et al. Occurrence of extended-spectrum b-lactamases in members of the family Enterobacteriaceae in Italy. Implications for Resistance to b-Lactams and Other Antimicrobial Drugs, 2002; 46: 196–202. doi:10.1128/AAC.46.1.196.
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Mental Health Findings, NSDUH Series H-47, HHS Publication No. (SMA) 13-4805. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
- Tacconelli E., De Angelis G., Frank U., et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect, 2014; 20 Suppl 1: 1–55. doi:10.1111/1469-0691.12427.
- Thu Trang N. H., Thieu Nga T. V., Farrar J., et al. The characterization of ESBL genes in Escherichia coli and Klebsiella pneumoniae causing nosocomial infections in Vietnam. J Infect Dev Ctries, 2013; 7: 922–928. doi:10.3855/jidc.2938.
- Warner M., Schenker N., Heinen M. A., Fingerhut L. A. The effects of recall on reporting injury and poisoning episodes in the National Health Interview Survey. Inj Prev, 2005; 11: 282–287. doi:10.1136/ip.2004.006965.


