Diversity of CD1a Positive Cells in Case of 25-hydroxyvitamin D Deficiency in Patients with Metabolic Syndrome
Diversity of CD1a Positive Cells in Case of 25-hydroxyvitamin D Deficiency in Patients with Metabolic Syndrome
Abstract
Introduction. Vitamin D has immunomodulatory properties and it affects the immune system through a number of mechanisms including the influence on dendritic cells (DCs). Langerhans cells (LC) are dendritic cells in epidermis and they belong to the skin immune system. DCs are professional antigen-presenting cells playing a major role in the induction of immune responses by activating native T cells. In literature, there are no reports regarding the influence of vitamin D on DCs in patients with metabolic syndrome.
The aim of the study is to explore the potential immunomodulatory activity of vitamin D on LC in case of metabolic syndrome.
Material and methods. In this study, we have analysed a group of patients of both genders with metabolic syndrome aged 40‒55 years. Patients’ clinical examinations, measurement of blood pressure and waist circumference were conducted. Blood biochemical analyses (cholesterol, HDL, LDL, vitamin D level) were determined. Full-thickness circular 4 mm Punch biopsies were taken in 49 patients. Specimens were stained by haematoxylin and eosin, as well as immunohistochemistry using a transmembrane CD1a Langerhans cells marker was done.
Results. Average age in both genders is 43 years, mean waist circumference – 95 cm, total cholesterol – 5.5 mmol/l, LDL – 2 mmol/l, average 25-hydroxyvitamin D – 27.0 ng/ml. In the skin conditioned with MS and low vitamin D level, evident perivascular accumulation of Langerhans cells (LC) in epidermis is observed; in some cases, there was diffuse mild interstitial cluster of LC. LC activity, amount and filling of them with Birbeck granules change in cases with 25-hydroxyvitamin D deficiency.
Conclusion. In patients with low 25-hydroxyvitamin D level, average LC quantity in one field of vision is higher in comparison with those, who have normal amount of 25-hydroxyvitamin D. A deeper investigation is necessary on the role of LC and vitamin D in case of metabolic syndrome in order to reveal their interaction with lymphocytes, plasma cells and mast cells as a part of skin immune system.
Introduction
The skin is the largest organ in the human body. A network composed of delicate physical, chemical, and immunological barriers in the skin makes it a perfect organ to protect the integrity of the human body. Anatomically, skin is divided into epidermis, dermis, and subcutaneous tissue, from the superficial to the deep tissues. Dendritic cells (DCs) are found in epidermis, which tend to migrate and drain in deeper layers to lymphatic system. DCs represent a heterogeneous cell population residing in most peripheral tissues, particularly at sites of interphase with the environment, e.g. skin and mucosa. They represent 1–3 % of the total cell numbers of these tissues [1].
In the periphery, immature DC (iDC) can capture and process antigens. Thereafter, they migrate towards T-cell-rich areas of the secondary lymphoid organs through the afferent lymphatic vessels.
Antigens are presented in such a way that antigen-specific native T cells become activated and start to proliferate. Moreover, when DC initiate a T-cell-mediated adaptive immune response, they also play an important role in polarization of T-cell reactivity [2].
In skin, these cells are dedicated antigen-presenting cells (APCs) and play a key role in sensing danger and initiating both innate and adaptive responses, as well as protect skin from invading pathogens.
The Langerhans cells have captured considerable interest since student Paul Langerhans first described them in 1865 as aureophilic cells present within the epidermis. At first, they were falsely described as melanocytic generation cells, due to their appearance. However, later, it was discovered that LC express MHC class I molecules [3], that indicates LC as important immunological antigen-presenting cells in the body [4]. LC represent approximately 1‒3 % of epidermal cells and form a constituent part of the skin immune system. LC are typically characterized by the expression of CD1a and a unique cytoplasmic organelle named the Birbeck granule (BG). BGs constitute a subdomain of the endosomal recycling compartment, perhaps being involved in antigen loading processing [5].
It has been postulated that bone-marrow-derived myeloid cutaneous lymphocyte-associated antigen (CLA)-expressing LC precursors travel through peripheral blood through the dermis into the epidermis. The final differentiation of LC precursors depends on the cytokine environment of the epidermis [5]. The cutaneous cytokines granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin- 15 (IL-15), and TGF-β1 contribute to the establishment of immature LC in the epidermis. During inflammation, however, circulating precursors might have an important role in replenishing the local pool of LC.
LC has different function according to location; thus in periphery immature LC capture and process antigens. In afferent lymphatic vessels, LC presents antigens and acquire the capacity to present antigens to native T cells (named as maturation of LC). Moreover, there are T cell activation and proliferation (named T cell priming), as well as polarization of T cell reactivity towards type 1 and / or type 2 responses. In epidermis and dermis, LC participate in recognition of invading pathogens (viral, bacterial, etc.), toxins and harmful irradiations. [6] In case of over expression of ultraviolet radiation (UV) from sun, LC might play role in skin immunosuppression. Under UV radiation in epidermis develop direct (intrinsic) keratinocyte damage via apoptosis with clustering of death receptors in the cell surface (extrinsic) and generation of reactive oxygen species (ROS). When apoptotic keratinocytes are processed by adjacent immature Langerhans cells, the inappropriately activated LC could result in immunosuppression. Furthermore, UV can deplete LC in the epidermis and impair its migratory capacity [7].
Vitamin D3 exerts pluripotent effects on adaptive immune functions such as T cell activation and maturation of dendritic cells. In addition, vitamin D3 is suggested to increase innate immunity in skin and to enable efficient antimicrobial defense at epithelial surfaces [8].
The surface of our skin is constantly being challenged by a wide variety of microbial pathogens; still cutaneous infections are relatively rare. Within cutaneous innate immunity, the production of antimicrobial peptides (AMPs) is a primary system for protection against infection. Many AMPs can be found in the skin and these include molecules that were discovered for their antimicrobial properties, and other peptides and proteins first known for activity as chemokines, enzymes, enzyme inhibitors and neuropeptides. Cathelicidins were among the first families of AMPs discovered on the skin. Now they are known to have two distinct functions: they have direct antimicrobial activity and initiate a host cellular response resulting in cytokine release, inflammation and angiogenesis [9].
Synthesis of pre-vitamin D3 from 7-dehydrocholesterol occurs in skin and involves UVB radiation that penetrates the epidermis. 7-dehydrocholesterol absorbs UV light most effectively at wavelengths between 270–290 nm and thus the production of vitamin D3 will occur at those wavelengths. Calciol, which is the product of the transformation of 7-dehydrocholesterol, is an inactive, unhydroxylated form of vitamin D3. To form the active hormone calciol must be hydroxylated twice to form calcidiol (25-hydroxyvitamin D3, 25D3) and finally, active calcitriol (1,25-dihydroxyvitamin D3, 1,25D3) (Figure 1). The two enzymes responsible for activating vitamin D3 – vitamin D 25-hydroxylase (CYP27A1) and 25-hydroxyvitamin D3. 1-α-hydroxylase (CYP27B1) were initially identified in liver and kidney [10, 11]. Keratinocytes express both enzymes and are capable of producing active 1,25D3 independent of renal and hepatic hydroxylation steps [12].
Vitamin D3 exerts a pluripotent effects on adaptive immune function by [13, 14]:
- adaptive T cell immune reaction;
- maturation and proliferation of LC from immature dendritic cells;
- increase of innate immunity in skin and regulation of antimicrobial defense at epithelial surfaces (cathelcidin expression);
- regulation of keratinocyte differentiation and proliferation, as well as production of intact epidermal barrier;
- changes in serum D vitamin level which may impact skin immune barrier functions and inflammatory reactions.
In skin, the presence of vitamin D3 is essential for normal keratinocyte development, differentiation and function [15]. Any alteration in local vitamin D3 concentrations and / or activation will likely affect normal cutaneous immune function, barrier function and inflammatory responses [16, 17].
The minimal concentration of vitamin D per day is well known – for adults who are not in risk group the amount is 1000 IU per day. In case of risk group (the elderly, dark skin, season, latitude, time of day, use of sunscreens, fat malabsorption, anticonvulsant use, chronic kidney disease, obesity) – at least 2000 IU is needed.
It has been reported that exposure of 6‒10 % of the body surface to 1 minimal erythemal dose, which is the minimum amount of UVB radiation that produce redness in 24 hours after exposure, is equivalent to ingesting about 600‒1000 IU of vitamin D [18].
Obesity is one of the risk factors for low vitamin D status; we investigated patients with metabolic syndrome. According to the International Diabetes Federation definition [Alberti, et al., 2006] a person can be characterized as having the metabolic syndrome if he has central obesity (waist circumference for women ≥ 80 cm, men ≥ 94 cm) and additionally two of any of the following factors: raised triglycerides, reduced HDL cholesterol, raised blood pressure, raised plasma glucose. In skin, metabolic syndrome causes a chronic latent inflammation that impact all skin functions, such as barrier, immune and endocrine function. Due to obesity, there are inadequate balance between high-density lipoproteins and low-density lipoproteins, that may induce defence skin immunological responses to external factors. The lipid abnormalities might facilitate and maintain the inflammatory reaction in the skin. Mainly, activity of macrophages and increased activity of matrix metalloproteinase are involved. There are no reports regarding the influence of vitamin D on DCs in MS patients.
The aim
The aim of our research is to explore the potential immunomodulatory activity of vitamin D on LC in case of metabolic syndrome.
Material and methods
Generally, 49 40‒55-year old patients of both genders have been analyzed (I-III Fitzpatrick’s skin phototype) in the Clinic of Dermatology, Rīga, Latvia. The respective study employed the group of patients with clinically and biochemically proved diagnosis of MS based on IDF 2006 criteria [International Diabetes Federation, 2006].
In the course of the study, several parameters were evaluated in all patients:
- clinical examination entailed measurement of the arterial blood pressure and waist circumference;
- instrumental examination of the skin surface with ARAMO SG – Skin Analyser (Aramhuvis Co) with 3 different lenses (× 1; × 10; × 60) for determining the moisture, sebum level, evenness of the surface, dyspigmentation, capillary characteristics and skin micro-relief;
- biochemical testing: total cholesterol level (TH), fasting plasma glucose, high density lipoproteins (HDL), low density lipoproteins (LDL) levels, 25-hydroxyvitamin D;
- full-thickness circular 4 mm Punch biopsies were obtained from the dorsal surface of the palm. Specimens were stained with haematoxylin-eosin and with Masson’s trichrome. Immunohistochemical antibody staining was conducted with Dako Cytomation EnVision system (Denmark) for the following antigens: CD34, CD117. Subsequently, capillaries and CD117 positive cells and fibres were quantified per 1 mm2. Axiostar Plus microscope with a ruler was used to measure adipocyte dimensions under × 400 magnification. CD1a positive Langerhans cells were evaluated in 3 fields of vision. The average quantity of CD1a positive Langerhans cells was calculated under × 400 magnification. All data were documented and analysed using Microsoft Excel 2012 software and MS Excel programmes.
Results
In the group of patients with MS, mean waist circumference for both genders was 95 cm. Average body mass index – 25.5 kg/m2. Women : men ratio – 1.6 : 1. Mean 25-hydroxyvitamin D minimal and maximal values are shown in Figure 1, assumed normal vitamin D level in serum in adults varies from 30 to 100 IU.
Regarding the blood biochemistry: average cholesterol level is 6.1 mmol/l, LDL – 3.5 mmol/l, HDL – 0.5 mmol/L, and CRP – 2.1 mg/L, respectively.
By dermatoscopic findings, in case of MS facial skin dyspigmentation and pronounced telangiectasia were revealed, yet skin micro-relief has not been altered.
In patients with MS, CD34 positive endothelial cells composed up to 4.5 capillaries per 1 mm2, in patients without MS, 3.1 capillaries per 1 mm2, respectively. The epidermal basal membrane was thickened in MS cases. CD117 positive mast cells were evenly distributed in the dermis and between adipocytes (8 CD117 positive cells per 1 mm2 on average).
Figure 1. 25-hydroxyvitamin D level in serum
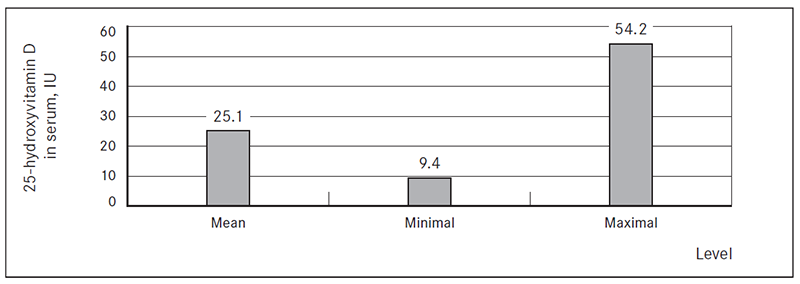
Table 1. Association of 25-hydroxyvitamin D level in serum and amount of LC in epidermis
| 25-hydroxyvitamin D in serum, IU | Average amount of LCs per field of vision |
|---|---|
| 8.3 | 18.0 |
| 10.3 | 25.0 |
| 20.8 | 13.1 |
| Up to 30 | 7.7 |
Immunoreactivity of bcl-2 protein had a patchy pattern in basal cells with a mean quantity of 39.1 per 100 basal cells in MS and 6.4 in persons without it. Average thickness of epidermis was 0.71 mm (patients with MS) and 0.46 mm (without MS). Thickness of stratum spinosum was 0.48 mm (patients with MS) and 0.25 mm (without MS). Thickness of stratum corneum was 0.18 mm (patients with MS) and 0.16 mm (without MS).
Enlargement of adipocytes up to 0.13 mm and fibrosis of deep vessels has been revealed.
Amount of DCs in epidermis varied from 32.6 to 13.5, the quantity per field of vision is illustrated in Table 1. In cases with low vitamin D level number of CD1a+ cells was 16.2 and there are tendency in increase of LC in epidermis (Figure 2).
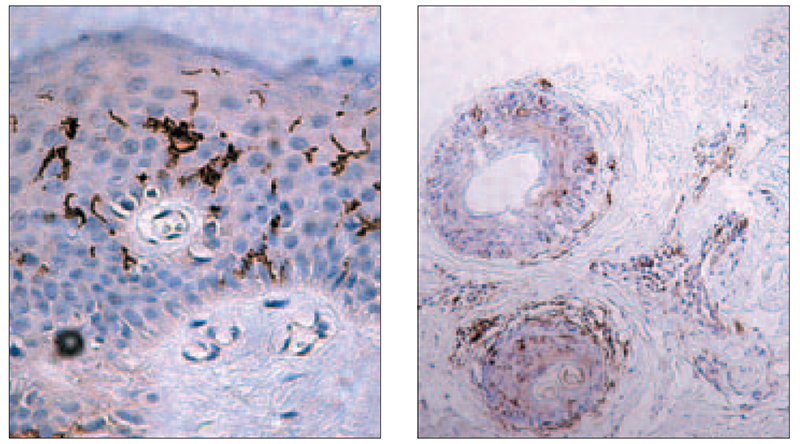
Significant accumulation of DCs around the cluster of inflammatory cells, acanthosis and hyperkeratosis were observed. Variable content of Birbeck granules in patients with MS was evaluated, as well as in some cases migration of CD 1a+ cells into papillary dermis (Figure 3). CD1a+ cells were revealed around apoptotic lesion in epidermis, as well as a mild inflammatory reaction around two skin appendages (Figure 4).
Figure 2. Migration of Langerhans cells into dermis (× 400, Dako)
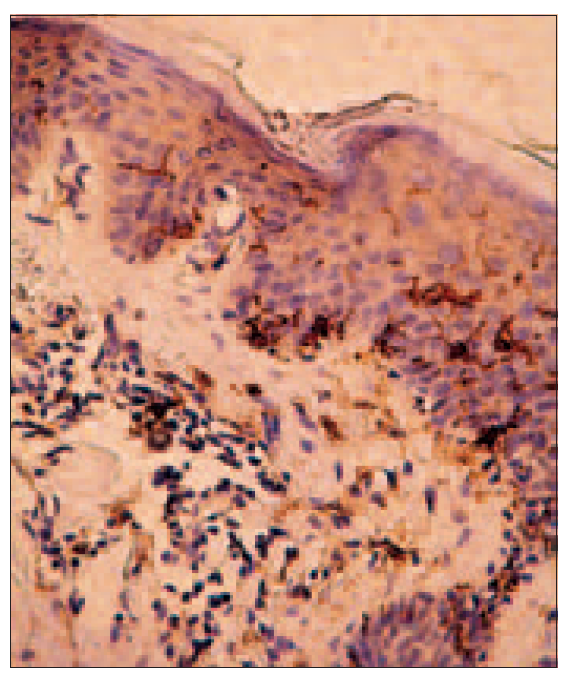
Figure 3. Adequate amount of LC amount (right) (× 100, Dako); increased number of LC in case of vitamin D deficiency (left) (× 400, Dako)
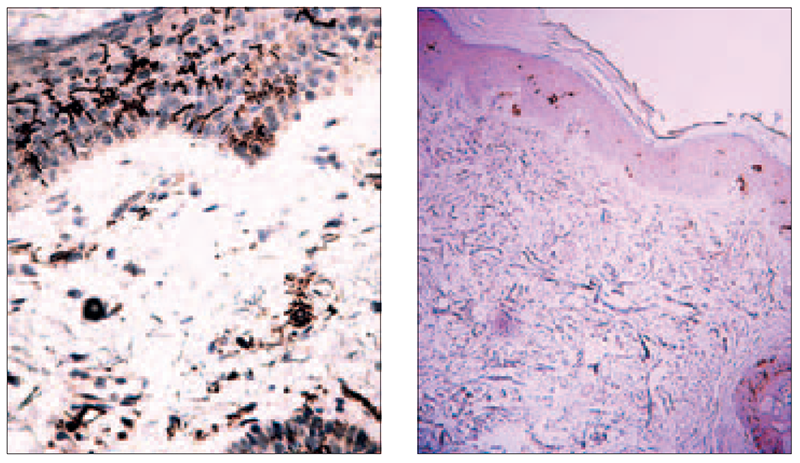
Figure 4. Concentration of CD1a+ cells around apoptotic keratinocytes (left) (× 400, Dako), mild inflammatory reaction and CD1a+ cells around appendages (right) (× 100, Dako)
Discussion
The immune system exists in a delicate equilibrium between inflammatory responses and tolerance. DCs are the primary professional antigen-presenting cells (APCs) initiating adaptive immune response. In this review, we summarize accumulating evidence about the role of regulatory DCs in situations where the balance between tolerance and immunogenicity has been altered leading to pathologic conditions such as diffuse chronic inflammation [19]. There are a lot of articles describing LC peculiarities in case of local skin lesion such as melanoma, basal cell carcinoma, etc. The role and characteristics of LC in basal cell carcinoma (BCC) is revealed. Thus, the lower density and fewer morphological changes of LC in the epidermis overlying BCC may give rise to alterations in the immune response to BCC [20]. I. Santos has described significant increase in the number of LC in the group with lower potential of local aggressiveness, as compared with the number of cells in the epidermis superposed to the basal cell carcinoma, thus limiting the aggressiveness of the neoplasm [21]. Patapova, et al. and Prignano, et al. underline the role of LC in skin chronic inflammatory diseases such as psoriasis and atopy, where LC participated in the reaction of allergic contact dermatitis and recognized them as antigen-presenting cells [22, 23].
As considered in case of MS, chronic latent inflammation and metabolic disturbances persist in the skin due to oxidative stress, that may affect skin immune system, as it was published before by J. Janovska, et al. [24]. Changes in CD3, CD20, CD8, accumulation of pro-apoptotic protein bcl-2, thickness of basal cell membrane of epidermis and mild inflammatory reaction around capillaries were revealed.
Our study showed that 25-hydroxyvitamin D deficiency in MS patients is associated with higher amount of dendritic cells and increase of their activity in epidermis. In patients with low 25-hydroxyvitamin D level in serum average LC quantity in 1 field of vision is higher (up to 25) compared with those, who have normal amount of 25-hydroxyvitamin D less than 10 CD1a positive cells. These peculiarities may be associated with skin immunomodulatory process and characterizes LC as antigen-presenting cells. Different amount of Birbeck’s granules filling is revealed, of those patients, whose skin is conducted with metabolic syndrome. In case of MS expansion of adipocytes and dermal fibrosis may point to hypoxia in tissue, which could be directly associated with presence of oxidative stress under metabolic syndrome.
Conclusions
- In patients with low vitamin D level in serum, average amount of Langerhans cells quantity in one field of vision is higher in comparison with those, who have sufficient rates of vitamin D.
- In patients with metabolic syndrome and vitamin D deficiency, Langerhans cells activity is higher and filling of Birbeck’s granules are more pronounced in comparison with those, who have sufficient vitamin D status.
- It is necessary to investigate more regarding Langerhans cells and vitamin D effect in case of metabolic syndrome in order to reveal more interaction with lymphocytes, plasma cells and mast cells as a part of skin immune system.
References
- Banchereau J., Steinman R. M. Dendritic cells and the control of immunity (Review) // Nature, 1998; 392: 245–252.
- Kissenpfennig A., Malissen B. Langerhans cells revisiting the paradigm using genetically engineered mice (Review) // Trends Immunol, 2006; 27: 132–139.
- Tony C., Ronald J. The normal Langerhans cell and the LCH cells // Br J Cancer, 1994; 70.
- Austyn J. M. Lymphoid dendritic cells // Immunology, 1987; 6: 161‒170.
- Erna J., Breve J., et al. Antigen-bearing Langerhans cells in skin draining lymph nodes: phenotype and kinetics of migration // J of Invest Dermatol, 1994: 217–220.
- Elbe A., Tschachler E., Steiner G., et al. Maturational steps of bone marrow-derived dendritic murine epidermal cells. Phenotypic and functional studies on Langerhans cells and Thy-1+ dendritic epidermal cells in the perinatal period // J Immunol, 1989; 143: 2431–2438.
- Schaerli P., Willimann K., Ebert L. M., et al. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation // Immunity, 2005; 23: 331–342.
- Toebak M. J., Gibbs S. Dendritic cells: biology of the skin // Contact Dermatitis, 2009; 60: 2‒20.
- Lee C. H., Wu S. B., Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy // Int J Mol Sci, 2013 Mar 20; 14 (3): 6414-35. doi: 10.3390/ijms14036414.
- Yim S., Dhawan P., Ragunath C., et al. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) // J Cyst Fibros, 2007; 6: 403–410.
- Schauber J., Dorschner R. A., Yamasaki K., et al. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli // Immunology, 2006; 118: 509–519.
- Prosser D. E., Jones G. Enzymes involved in the activation and inactivation of vitamin D // Trends Biochem Sci, 2004; 29: 664–673.
- Dixon K. M., Tongkao-on W. Vitamin D and death by sunshine // Int J Mol Sci, 2013; 18; 14 (1): 1964‒1977.
- Van Etten E., Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts // J Steroid Biochem Mol Biol, 2005; 97: 93–101.
- Schauber J., et al. Antimicrobial peptides and the skin immune defense system // J Aller Clin Imunol, 2009; 124 (3).
- Yim S., Dhawan P., et al. Induction of cathelicidin in normal and CSF bronchial epithelial cells by D(3) // J Cyst Fibros, 2007; 6: 403‒410.
- Bikle D. D. Vitamin D regulated keratinocyte differentiation // J Cell Biochem, 2004; 92: 436–444.
- Oda Y., Sihlbom C., Chalkley R. J., et al. Two distinct co-activators, DRIP/mediator and SRC/p160, are differentially involved in VDR transactivation during keratinocyte differentiation // J Steroid Biochem Mol Biol, 2004; 90: 273–276.
- Bikle D., Chang S., Crumrine D., et al. Mice lacking 25OHD 1alpha-hydroxylase demonstrate decreased epidermal differentiation and barrier function // J Steroid Biochem Mol Biol, 2004; 5: 347–353.
- Ran Zhang, Naughton D. P. Vitamin D in health and disease // Nutrition Journal, 2010; 9: 65.
- Schmidt S., Andrea C., et al. Regulatory dendritic cells: there is more than just immune activation // J Frontiers in Immunology, 2012; 3: 274.
- Mardones F., Zemelman V., Sazunic I., et al. CD1a+ Langerhans cells in the peritumoral epidermis of basal cell carcinoma // Actas Dermosifiliogr, 2009; 100: 700‒705.
- Santos I., Mello R. J., Santos I. B., et al. Quantitative study of Langerhans cells in basal cell carcinoma with higher or lower potential of local aggressiveness // An Bras Dermatol, 2010 Mar‒Apr; 85 (2): 165‒171.
- Potapova O., Luzgina N., Shkurupiy V. Immunomorphological study of Langerhans cells in skin of patients with atopic dermatitis // Bull Exp Biol Med, 2008; 146 (6); 809‒811.
- Prignano F., Gerlini G., Fossombroni V., et al. Control of the differentiation state and function of human epidermal Langerhans cells by cytokines in vitro // J Eur Acad Dermatol Venerol, 2001 Sept; 15 (5): 433‒440.
- Janovska J., Kisis J., Voicehovska J., et al. Pilot study of early skin changes due to metabolic syndrome // Collection of scietific papers 2012. – Rīga: RSU, 2013. – Pp. 11‒17.


